Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
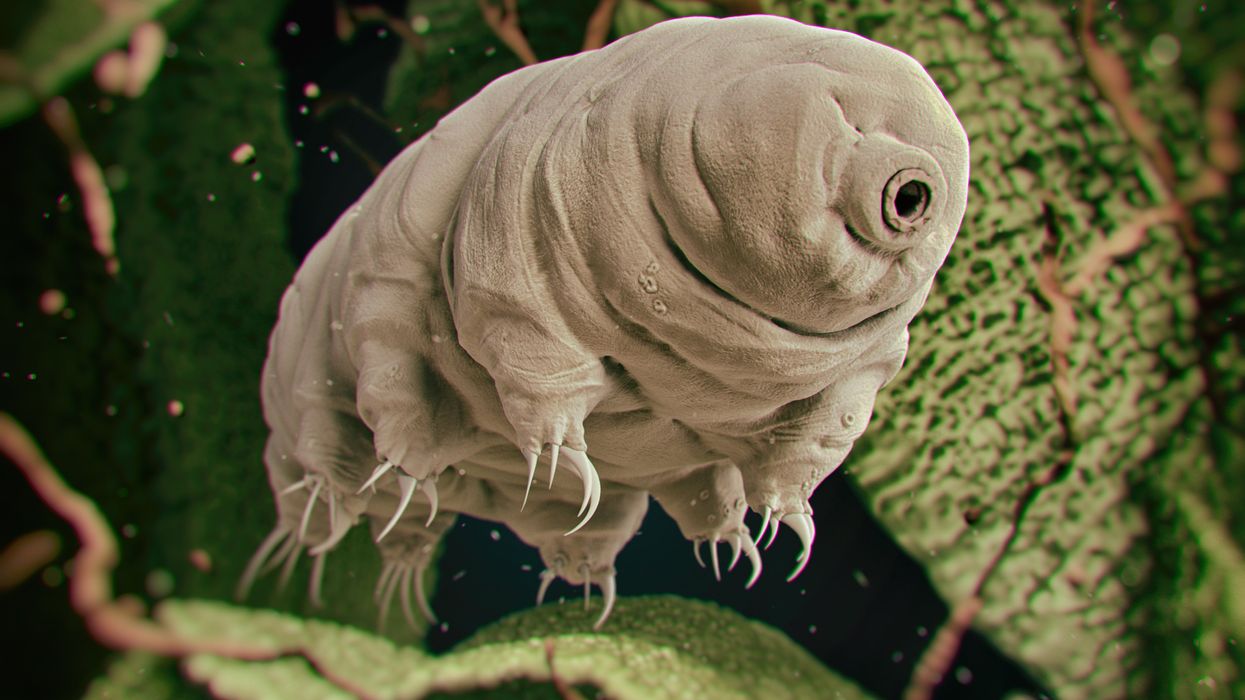
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Vaccines Without Vaccinations Won’t End the Pandemic
In this 2020 photograph, a bandage is placed on a patient who has just received a vaccine.
COVID-19 vaccine development has advanced at a record-setting pace, thanks to our nation's longstanding support for basic vaccine science coupled with massive public and private sector investments.
Yet, policymakers aren't according anywhere near the same level of priority to investments in the social, behavioral, and data science needed to better understand who and what influences vaccination decision-making. "If we want to be sure vaccines become vaccinations, this is exactly the kind of work that's urgently needed," says Dr. Bruce Gellin, President of Global Immunization at the Sabin Vaccine Institute.
Simply put: it's possible vaccines will remain in refrigerators and not be delivered to the arms of rolled-up sleeves if we don't quickly ramp up vaccine confidence research and broadly disseminate the findings.
According to the most recent Gallup poll, the share of U.S. adults who say they would get a COVID-19 vaccine rose to 58 percent this month from 50 percent in September, with non-white Americans and those ages 45-65 even less willing to be vaccinated. While there is still much we don't understand about COVID-19, we do know that without high levels of immunity in the population, a return to some semblance of normalcy is wishful thinking.
Research from prior vaccination campaigns such as H1N1, HPV, and the annual flu points us in the right direction. Key components of successful vaccination efforts require 1) Identifying the concerns of particular segments of the population; 2) Tailoring messages and incentives to address those concerns, and 3) Reaching out through trusted sources – health care providers, public health departments, and others in the community.
Research during the H1N1 flu found preparing people for some uncertainty actually improved trust, according to Dr. Sandra Crouse Quinn, professor and chair, Family Science, University of Maryland. Dr. Crouse Quinn's research during that period also underscored the need to address the specific vaccine concerns of racial and ethnic groups.
The stunning scientific achievement of COVID-19 vaccines anticipated to be ready in record time needs to be backed up by an equally ambitious and evidence-based effort to build the public's confidence in the vaccines.
Data science has provided crucial insight about the social media universe. Dr. Neil Johnson, a scientist at George Washington University, found that despite having fewer followers, anti-vaccination pages are more numerous and growing faster than pro-vaccination pages. They are more often linked to in discussions on other Facebook pages – such as school parent associations – where people are undecided about vaccination.
We've learned about building vaccine confidence from earlier campaigns. Now, however, we are faced with a unique and challenging set of obstacles to unpack quickly: How do we communicate the importance of eventual COVID-19 vaccines to Americans in light of the muddled-to-poor messaging from political leaders, the weaponizing of relatively simple public health recommendations, the enormous disproportionate toll on people of color, and the torrent of online misinformation? We urgently need data reflective of today's circumstances along with the policy to ensure it is quickly and effectively disseminated to the public health and clinical workforce.
Last year prompted in part by the measles outbreaks, Reps. Michael C. Burgess (R-TX) and Kim Shrier (D-WA), both physicians, introduced the bipartisan Vaccines Act to develop a national surveillance system to monitor vaccination rates and conduct a national campaign to increase awareness of the importance of vaccines. Unfortunately, that legislation wasn't passed. In response to COVID-19, Senate HELP Committee Ranking member Patty Murray (D-WA) has sought funds to strengthen vaccine confidence and combat misinformation with federally supported communication, research, and outreach efforts. Leading experts outside of Congress have called for this type of research, including the Sabin-Aspen Vaccine Science Policy Institute. Most recently, the National Academy of Sciences, in its report regarding the equitable distribution of the COVID-19 vaccine, included as one of its recommendations the need for "a rapid-response program to advance the science behind vaccine confidence."
Addressing trust in vaccination has never been as challenging nor as consequential. The stunning scientific achievement of COVID-19 vaccines anticipated to be ready in record time needs to be backed up by an equally ambitious and evidence-based effort to build the public's confidence in the vaccines. In its remaining days, the Trump Administration should invest in building vaccine confidence with current resources, targeting efforts to ensure COVID vaccines reduce rather than exacerbate racial and ethnic health disparities. Congress must also act to provide the additional research and outreach resources needed as well as pass the Vaccines Act so we are better prepared in the future.
If we don't succeed, COVID-19 will continue wreaking havoc on our health, our society, and our economy. We will also permanently jeopardize public trust in vaccines – one of the most successful medical interventions in human history.
New Video: Secret Heroes of the Pandemic
These 6 people deserve to be widely known and celebrated for their brave and ingenious contributions to battling the pandemic.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.