Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
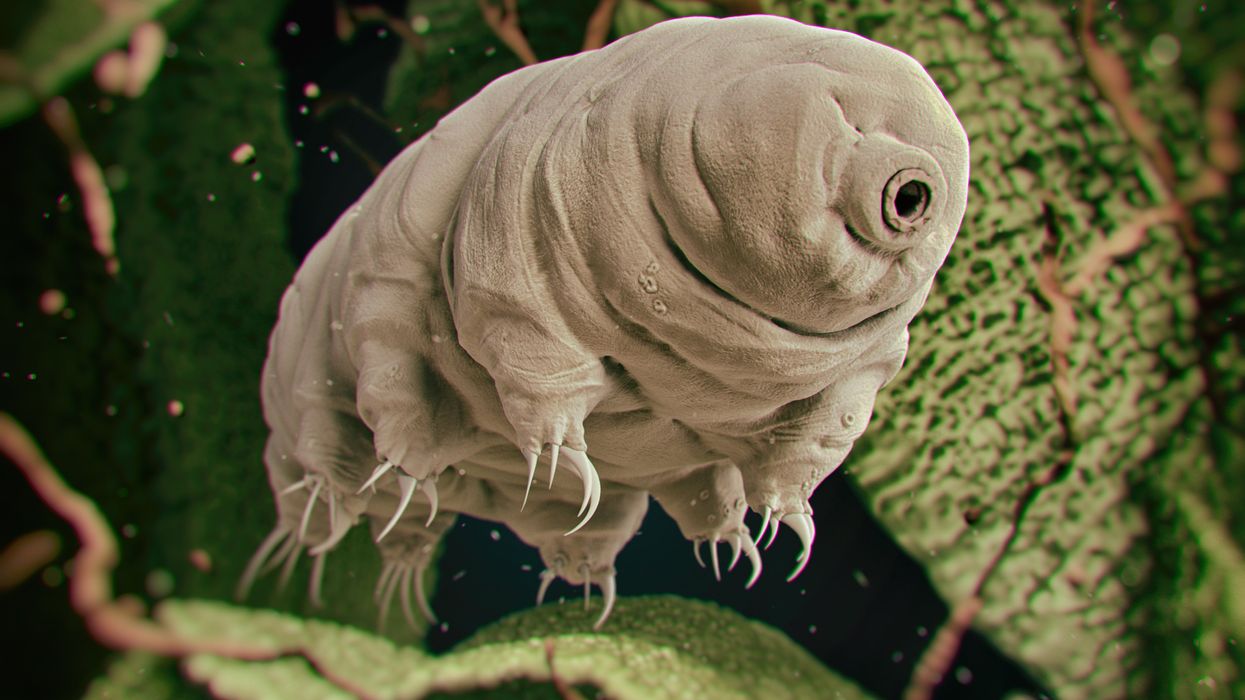
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
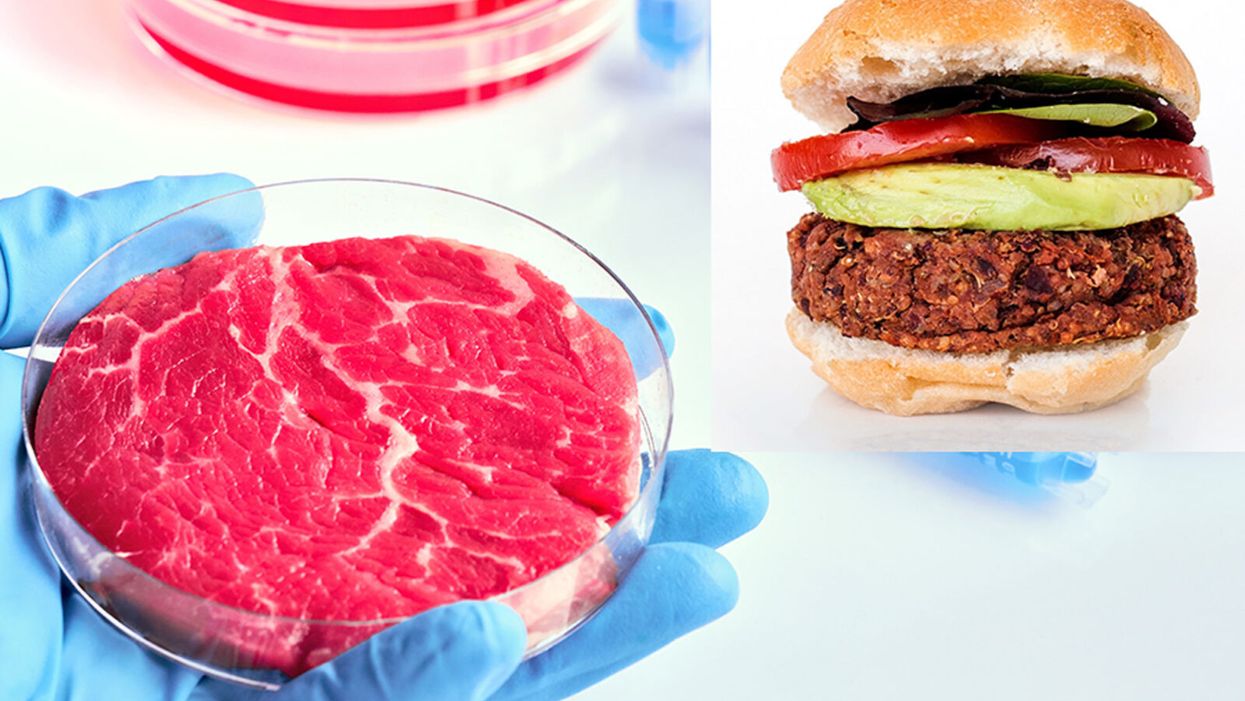
While lab-grown meat is not yet ready to crisis-proof the food supply chain, it offers key benefits that could make it an important solution beyond this pandemic.
The coronavirus pandemic exposed significant weaknesses in the country's food supply chain. Grocery store meat counters were bare. Transportation interruptions influenced supply. Finding beef, poultry, and pork at the store has been, in some places, as challenging as finding toilet paper.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle.
It wasn't a lack of supply -- millions of animals were in the pipeline.
"There's certainly enough food out there, but it can't get anywhere because of the way our system is set up," said Amy Rowat, an associate professor of integrative biology and physiology at UCLA. "Having a more self-contained, self-sufficient way to produce meat could make the supply chain more robust."
Cultured meat could be one way of making the meat supply chain more resilient despite disruptions due to pandemics such as COVID-19. But is the country ready to embrace lab-grown food?
According to a Good Food Institute study, GenZ is almost twice as likely to embrace meat alternatives for reasons related to social and environmental awareness, even prior to the pandemic. That's because this group wants food choices that reflect their values around food justice, equity, and animal welfare.
Largely, the interest in protein alternatives has been plant-based foods. However, factors directly related to COVID-19 may accelerate consumer interest in the scaling up of cell-grown products, according to Liz Specht, the associate director of science and technology at The Good Food Institute. The latter is a nonprofit organization that supports scientists, investors, and entrepreneurs working to develop food alternatives to conventional animal products.
While lab-grown food isn't ready yet to definitively crisis-proof the food supply chain, experts say it offers promise.
Matching Supply and Demand
Companies developing cell-grown meat claim it can take as few as two months to develop a cell into an edible product, according to Anthony Chow, CFA at Agronomics Limited, an investment company focused on meat alternatives. Tissue is taken from an animal and placed in a culture that contains nutrients and proteins the cells need to grow and expand. He cites a Good Food Institute report that claims a 2.5-millimeter sample can grow three and a half tons of meat in 40 days, allowing for exponential growth when needed.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle. To keep enough maturing animals in the pipeline, farms must plan the number of animals to raise months -- even years -- in advance. Lab-grown meat advocates say that because cultured meat supplies can be flexible, it theoretically allows for scaling up or down in significantly less time.
"Supply and demand has drastically changed in some way around the world and cultivated meat processing would be able to adapt much quicker than conventional farming," Chow said.
Scaling Up
Lab-grown meat may provide an eventual solution, but not in the immediate future, said Paul Mozdziak, a professor of physiology at North Carolina State University who researches animal cell culture techniques, transgenic animal production, and muscle biology.
"The challenge is in culture media," he said. "It's going to take some innovation to get the cells to grow at quantities that are going to be similar to what you can get from an animal. These are questions that everybody in the space is working on."
Chow says some of the most advanced cultured meat companies, such as BlueNal, anticipate introducing products to the market midway through next year. However, he thinks COVID-19 has slowed the process. Once introduced, they will be at a premium price, most likely available at restaurants before they hit grocery store shelves.
"I think in five years' time it will be in a different place," he said. "I don't think that this will have relevance for this pandemic, but certainly beyond that."
"Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
Of course, all the technological solutions in the world won't solve the problem unless people are open-minded about embracing them. At least for now, a lab-grown burger or bluefin tuna might still be too strange for many people, especially in the U.S.
For instance, a 2019 article published by "Frontiers in Sustainable Food Systems" reflects results from a study of 3,030 consumers showing that 29 percent of U.S. customers, 59 percent of Chinese consumers, and 56 percent of Indian consumers were either 'very' or 'extremely likely' to try cultivated meat.
"Lab-grown meat is genuine meat, at the cellular level, and therefore will match conventional meat with regard to its nutritional content and overall sensory experience. It could be argued that plant-based meat will never be able to achieve this," says Laura Turner, who works with Chow at Agronomics Limited. "Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
A Solution Beyond This Pandemic
The coronavirus has done more than raise awareness of the fragility of food supply chains. It has also been a wakeup call for consumers and policy makers that it is time to radically rethink our meat, Specht says. Those factors have elevated the profile of lab-grown meat.
"I think the economy is getting a little bit more steam and if I was an investor, I would be getting excited about it," adds Mozdziak.
Beyond crises, Mozdziak explains that as affluence continues to increase globally, meat consumption increases exponentially. Yet farm animals can only grow so quickly and traditional farming won't be able to keep up.
"Even Tyson is saying that by 2050, there's not going to be enough capacity in the animal meat space to meet demand," he notes. "If we don't look at some innovative technologies, how are we going to overcome that?"
A COVID-19 moonshot program challenged chemists around the world to submit ideas for how best to design a drug to target the virus.
By mid-March, Alpha Lee was growing restless. A pioneer of AI-driven drug discovery, Lee leads a team of researchers at the University of Cambridge, but his lab had been closed amidst the government-initiated lockdowns spreading inexorably across Europe.
If the Moonshot proves successful, they hope it could serve as a future benchmark for finding new medicines for chronic diseases.
Having spoken to his collaborators across the globe – many of whom were seeing their own experiments and research projects postponed indefinitely due to the pandemic – he noticed a similar sense of frustration and helplessness in the face of COVID-19.
While there was talk of finding a novel treatment for the virus, Lee was well aware the process was likely to be long and laborious. Traditional methods of drug discovery risked suffering the same fate as the efforts to find a cure for SARS in the early 2000, which took years and were ultimately abandoned long before a drug ever reached the market.
To avoid such an outcome, Lee was convinced that global collaboration was required. Together with a collection of scientists in the UK, US and Israel, he launched the 'COVID Moonshot' – a project which encouraged chemists worldwide to share their ideas for potential drug designs. If the Moonshot proves successful, they hope it could serve as a future benchmark for finding new medicines for chronic diseases.
Solving a Complex Jigsaw
In February, ShanghaiTech University published the first detailed snapshots of the SARS-CoV-2 coronavirus's proteins using a technique called X-ray crystallography. In particular, they revealed a high-resolution profile of the virus's main protease – the part of its structure that enables it to replicate inside a host – and the main drug target. The images were tantalizing.
"We could see all the tiny pieces sitting in the structure like pieces of a jigsaw," said Lee. "All we needed was for someone to come up with the best idea of joining these pieces together with a drug. Then you'd be left with a strong molecule which sits in the protease, and stops it from working, killing the virus in the process."
Normally, ideas for how best to design such a drug would be kept as carefully guarded secrets within individual labs and companies due to their potential value. But as a result, the steady process of trial and error to reach an optimum design can take years to come to fruition.
However, given the scale of the global emergency, Lee felt that the scientific community would be open to collective brainstorming on a mass scale. "Big Pharma usually wouldn't necessarily do this, but time is of the essence here," he said. "It was a case of, 'Let's just rethink every drug discovery stage to see -- ok, how can we go as fast as we can?'"
On March 13, he launched the COVID moonshot, calling for chemists around the globe to come up with the most creative ideas they could think of, on their laptops at home. No design was too weird or wacky to be considered, and crucially nothing would be patented. The entire project would be done on a not-for-profit basis, meaning that any drug that makes it to market will have been created simply for the good of humanity.
It caught fire: Within just two weeks, more than 2,300 potential drug designs had been submitted. By the middle of July, over 10,000 had been received from scientists around the globe.
The Road Toward Clinical Trials
With so many designs to choose from, the team has been attempting to whittle them down to a shortlist of the most promising. Computational drug discovery experts at Diamond and the Weizmann Institute of Science in Rehovot, Israel, have enabled the Moonshot team to develop algorithms for predicting how quick and easy each design would be to make, and to predict how well each proposed drug might bind to the virus in real life.
The latter is an approach known as computational covalent docking and has previously been used in cancer research. "This was becoming more popular even before COVID-19, with several covalent drugs approved by the FDA in recent years," said Nir London, professor of organic chemistry at the Weizmann Institute, and one of the Moonshot team members. "However, all of these were for oncology. A covalent drug against SARS-CoV-2 will certainly highlight covalent drug-discovery as a viable option."
Through this approach, the team have selected 850 compounds to date, which they have manufactured and tested in various preclinical trials already. Fifty of these compounds - which appear to be especially promising when it comes to killing the virus in a test tube – are now being optimized further.
Lee is hoping that at least one of these potential drugs will be shown to be effective in curing animals of COVID-19 within the next six months, a step that would allow the Moonshot team to reach out to potential pharmaceutical partners to test their compounds in humans.
Future Implications
If the project does succeed, some believe it could open the door to scientific crowdsourcing as a future means of generating novel medicine ideas for other diseases. Frank von Delft, professor of protein science and structural biology at the University of Oxford's Nuffield Department of Medicine, described it as a new form of 'citizen science.'
"There's a vast resource of expertise and imagination that is simply dying to be tapped into," he said.
Others are slightly more skeptical, pointing out that the uniqueness of the current crisis has meant that many scientists were willing to contribute ideas without expecting any future compensation in return. This meant that it was easy to circumvent the traditional hurdles that prevent large-scale global collaborations from happening – namely how to decide who will profit from the final product and who will hold the intellectual property (IP) rights.
"I think it is too early to judge if this is a viable model for future drug discovery," says London. "I am not sure that without the existential threat we would have seen so many contributions, and so many people and institutions willing to waive compensation and future royalties. Many scientists found themselves at home, frustrated that they don't have a way to contribute to the fight against COVID-19, and this project gave them an opportunity. Plus many can get behind the fact that this project has no associated IP and no one will get rich off of this effort. This breaks down a lot of the typical barriers and red-tape for wider collaboration."
"If a drug would sprout from one of these crowdsourced ideas, it would serve as a very powerful argument to consider this mode of drug discovery further in the future."
However the Moonshot team believes that if they can succeed, it will at the very least send a strong statement to policy makers and the scientific community that greater efforts should be made to make such large-scale collaborations more feasible.
"All across the scientific world, we've seen unprecedented adoption of open-science, collaboration and collegiality during this crisis, perhaps recognizing that only a coordinated global effort could address this global challenge," says London. "If a drug would sprout from one of these crowdsourced ideas, it would serve as a very powerful argument to consider this mode of drug discovery further in the future."
[An earlier version of this article was published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]