Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
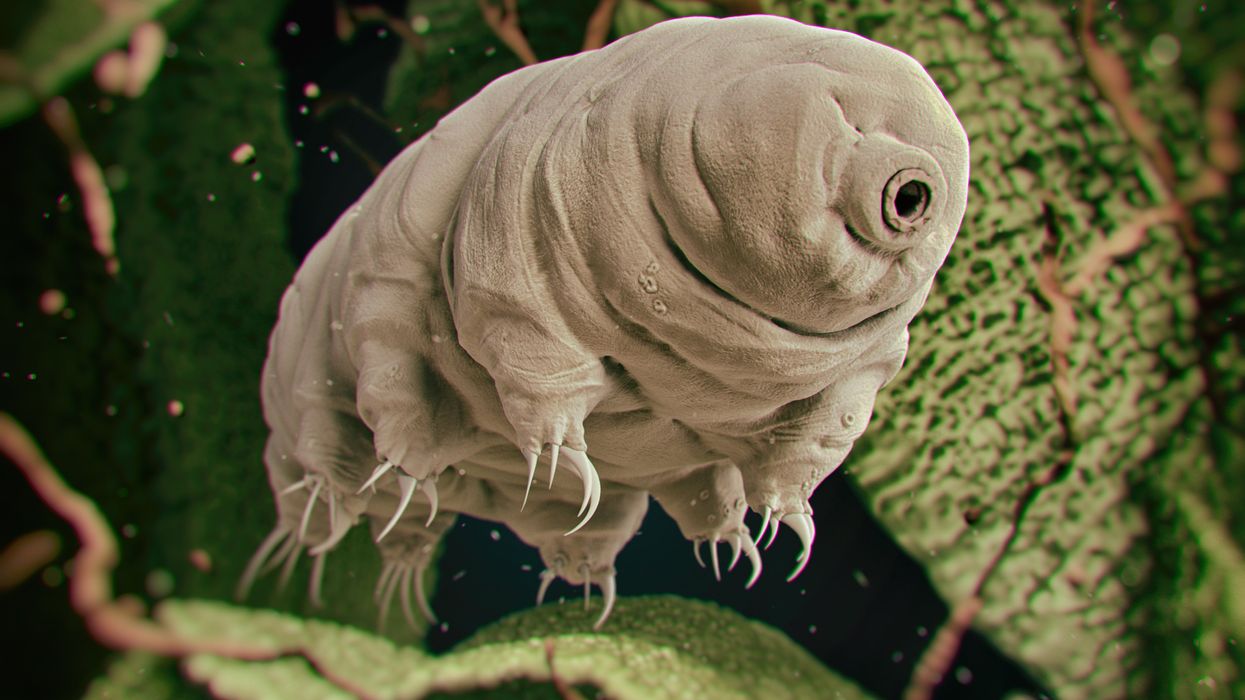
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
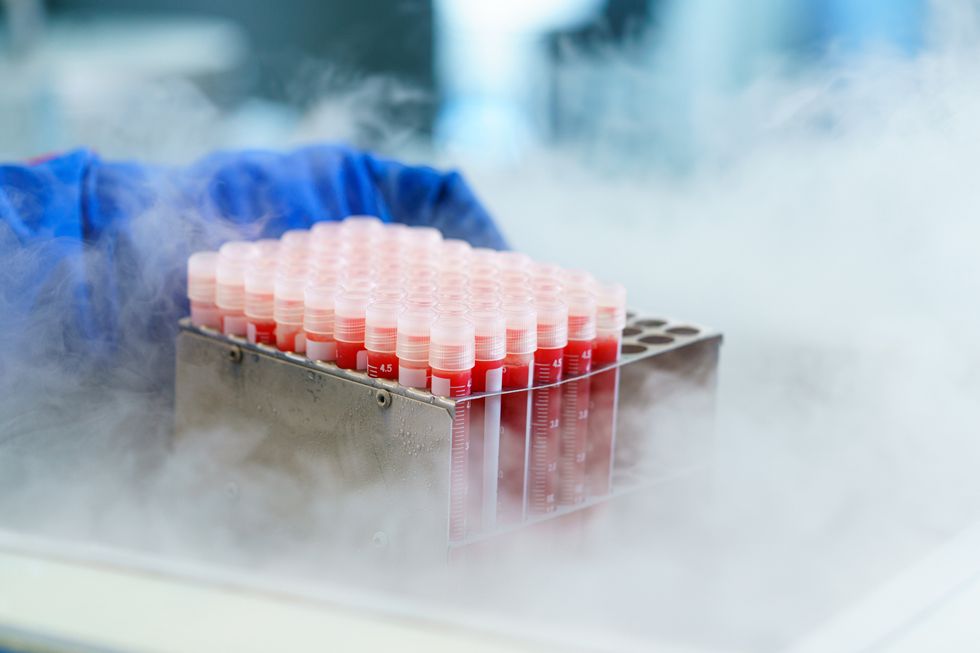
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Due to federal regulations, access to abortion medications is restricted, despite their record of safety and efficacy.
A few days before Christmas 2015, Paige Alexandria, a 28-year-old counselor at the Austin Women's Health Center in Texas, found out she was pregnant.
Alexandria had missed the cutoff for a medication abortion by three days.
"It was an unplanned pregnancy, and instantaneously I knew I needed an abortion," Alexandria recalls. Already a mother of two children, one with special needs, a third child was not something Alexandria and her husband felt prepared to take on. "Mentally, I knew my limit. I wasn't prepared for a third and I didn't want one," she says.
At an ultrasound appointment one week later, scans showed she was a little over eight weeks pregnant. Alexandria opted to have an abortion as soon as possible, and preferably with medication. "I really wanted to avoid a surgical abortion," she says. "It sounded a lot more invasive, and I'm already uncomfortable with pap smears and pelvic exams, so I initially went in wanting to do the pill."
But at the time, medication guidelines stipulated that one of the pills, called Mifepristone, could only be prescribed to end a pregnancy at eight weeks gestation or earlier – Alexandria had missed the cutoff by three days. If she wanted to end the pregnancy, she would need to undergo a surgical abortion, otherwise known as a vacuum aspiration abortion.
With a vacuum aspiration abortion, doctors dilate the cervix and manually aspirate out the contents of the uterus. Medication abortion, on the other hand, consists of the patient taking two pills – Mifepristone, which blocks the hormones that help the pregnancy develop, and Misoprostol, which empties the uterus over a period of days, identical to a miscarriage.
Alexandria was upset about the change of plans but resolute in her decision to end the pregnancy. "The fact that I didn't really have a choice in how my procedure was performed has made the experience just a little more sensitive for me," she says. She scheduled the earliest available appointment for a surgical abortion.

Paige Alexandria would have chosen to terminate her pregnancy with medication if the regulations were less stringent.
(Photo courtesy of Alexandria)
Like Alexandria, many people looking to terminate a pregnancy opt to do so with medication. According to research from the Guttmacher Institute, medication abortions accounted for nearly 40 percent of all abortions in the year 2017 – a marked increase from 2001, when medication abortions only accounted for roughly five percent of terminations. Taken 24-48 hours apart, Mifepristone and Misoprostol have a 95-99 percent success rate in terminating pregnancies up to 63 days – or nine weeks – of gestation, according to the American College of Obstetrics and Gynecology (ACOG).
But even though the World Health Organization (WHO) considers medical abortion to be highly safe and effective, the medication is still carefully guarded in the United States: Mifepristone is only available for terminating pregnancies up to 10 weeks gestation, per the FDA, even though limited research suggests that both are safe and effective at terminating pregnancies between 12 and 20 weeks.
Additionally, a separate set of regulations known as a Risk Evaluation and Mitigation Strategy (REMS) means that patients can only take Mifepristone under specific circumstances. Mifepristone must be distributed in person by a healthcare provider – usually interpreted in most states as a doctor or nurse practitioner – who has registered with the drug's manufacturer. The medication cannot be distributed through a pharmacy, so doctors who wish to provide the drug must stock the medication in-office, and both the provider and the patient must sign a form that warns them of the "risk of serious complications associated with Mifepristone," according to the FDA.
"REMS is a set of restrictions that the FDA puts on the distribution of drugs it considers dangerous or risky in some way," says Dr. Elizabeth Raymond, an OB-GYN and senior medical associate at Gynuity Health Projects. Although not always called REMS, these restrictions have been imposed on Mifepristone since the medication was approved by the FDA in 2000, Raymond says.
Raymond is part of a growing number of physicians and researchers who want to eliminate the REMS requirements for Mifepristone, also known by its brand name Mifeprex. In 2017, Raymond and several other physicians authored a paper in the New England Journal of Medicine (NEJM) arguing that Mifepristone is extremely safe and needlessly over-regulated.
"When the FDA first approved [Mifepristone] and imposed these requirements, they might have made sense 19 years ago when there was limited information about the use of this treatment in the United States," says Dr. Daniel Grossman, director at Advancing New Standards in Reproductive Health at UCSF and co-author of the 2017 report in the NEJM. "Now, after 19 years, it's clear that this medication is very safe, and safer than a lot of others available in a pharmacy."
Since 2000, Mifepristone has been implicated in 19 deaths, making its mortality rate 0.00063 percent.
According to their research, over three million people have taken Mifepristone since it was approved in 2000. Since then, Mifepristone has been implicated in 19 deaths, making its mortality rate 0.00063 percent. Even then, the risk is inflated, Grossman says.
"The requirement is that practitioners need to report any deaths that occur after taking these medications, and so you'll see deaths included in that figure which are homicides or suicides or something unrelated to taking Mifepristone," says Grossman. In contrast, Acetaminophen – better known as Tylenol – was associated with 458 overdose deaths between 1990 and 1998, as well as 56,000 emergency room visits and 26,000 hospitalizations. Sildenafil, better known as Viagra, was linked to 762 deaths in the first twenty months after it was approved by the FDA. Yet neither Tylenol nor Viagra have been burdened with the same REMS restrictions as Mifepristone.
"It's clearly about more than just the safety of the medication at this point," says Grossman. "It's more about stigma related to abortion and politics."
For people who want a medication abortion, the REMS requirements mean they often need to take off work to schedule a doctor's appointment, arrange for transportation and childcare, and then arrange an additional doctor's appointment days afterward to take the second dose of medication. While surgical abortion procedures are quicker (usually a one-day outpatient procedure, depending on gestation), many people prefer having the abortion in the comfort of their home or surrounded by family instead.
Paige Alexandria, who counsels people seeking abortions at her job, says that survivors of sexual violence often prefer medical abortions to surgical ones. "A lot of time survivors have a trauma associated with medical instruments or having pelvic exams, and so they're more comfortable taking a pill," she says.
But REMS also creates a barrier for healthcare providers, Grossman says. Stocking the medication in-office is "a hassle" and "expensive," while others are reluctant to register their name with the drug manufacturer, fearing harassment or violence from anti-choice protestors. As a result, the number of practitioners willing to provide medical abortions nationwide is severely limited. According to Grossman's own research published in the journal Obstetrics and Gynecology, 28 percent of OBGYNs admitted they would administer medication abortions if it were possible to write a prescription for Mifepristone rather than stock it in-office.
Amazingly, the restrictions on Mifepristone have loosened since it first came on the market. In 2016, the FDA updated the guidelines on Mifepristone to allow its use until 10 weeks gestation, up from eight weeks. But doctors say the REMS restrictions should be eliminated completely so that people can obtain abortions as early as possible.
"REMS restrictions inhibit people from being able to get a timely abortion," says Raymond, who stresses that abortion is generally more comfortable, more affordable, and safer for women the earlier it's done. "Abortion is very safe no matter when you get it, but it's also easier because there's less risk for bleeding, infections, or other complications," Raymond says. Abortions that occur earlier than eight weeks of gestation have a complication rate of less than one percent, while an abortion done at 12 or 13 weeks has a three to six percent chance of complications.
And even for people who want a medication abortion early on in their pregnancy, REMS restrictions make it so that they may not have time to obtain it before the 10-week period lapses, Raymond says.
"If you're seven weeks pregnant but it takes you three weeks to figure out travel and childcare arrangements to go into the doctor and take this medication, now you're at the cutoff date," she says. "Even if you manage to get an abortion at nine weeks, that's still a later gestational age, and so the risks are increased."
In 2016, at a little over nine weeks gestation, Alexandria completed her abortion by having a D&E. But because she didn't have anyone to drive her home after the procedure, she wasn't able to have sedation throughout, something she describes as "traumatic."
"I had the abortion completely aware and coherent, and paired with the fact that I hadn't even wanted a surgical abortion in the first place made it harder to deal with," Alexandria says.
"When you're just a day or two past eight weeks and you want an abortion – why is medication not immediately available?"
Today, Alexandria shares her story publicly to advocate for abortion care. Although she doesn't regret her surgical abortion and acknowledges that not everyone experiences surgical abortion the same way she did, she does wish that she could have gone a different route.
"If I had to do it over, I would still try to do the pill, because [the surgical abortion] was such a terrifying experience," she says. "When you're just a day or two past eight weeks and you want an abortion – why is medication not immediately available? It just doesn't make sense."
How Scientists Are Engineering Plastic-Eating Bacteria to Fight the Pollution Crisis
Scientists are turning to synthetic biology to engineer bacteria that can degrade plastic and turn it into higher-value materials.
[Ed. Note: This is the second episode in our Moonshot series, which will explore four cutting-edge scientific developments that stand to fundamentally transform our world.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

