Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
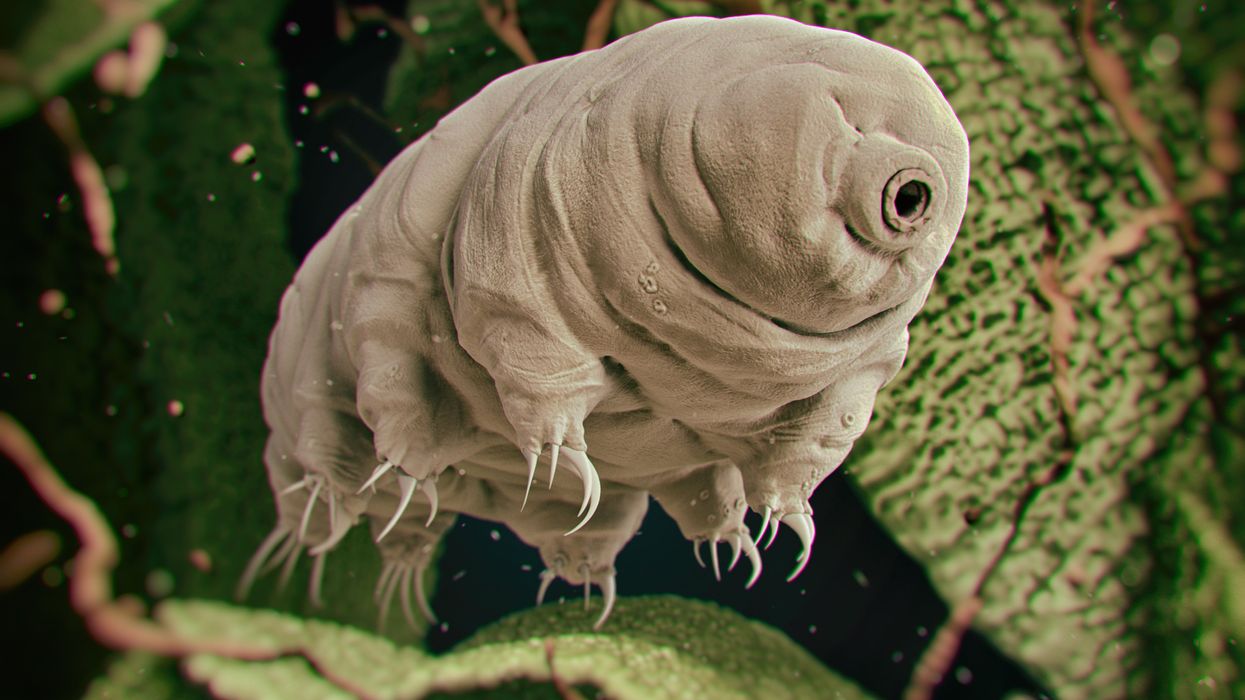
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
The field of treating schizophrenia with drugs has been stuck in a long drought but, this month, a late-stage clinical trial found a new drug called KarXT could treat a range of symptoms.
Schizophrenia is a debilitating mental health condition that affects around 24 million people worldwide. Patients experience hallucinations and delusions when they develop schizophrenia, with experts referring to these new thoughts and behaviors as positive symptoms. They also suffer from negative symptoms in which they lose important functions, suffering from dulled emotions, lack of purpose and social withdrawal.
Currently available drugs can control only a portion of these symptoms but, on August 8th, Karuna Therapeutics announced its completion of a phase 3 clinical trial that found a new drug called KarXT could treat both positive and negative symptoms of schizophrenia. It could mean substantial progress against a problem that has stymied scientists for decades.
A long-standing problem
Since the 1950s, antipsychotics have been used to treat schizophrenia. People who suffer from it are thought to have too much of a brain chemical called dopamine, and antipsychotics work by blocking dopamine receptors in the brain. They can be effective in treating positive symptoms but have little impact on the negative ones, which can be devastating for a patient’s quality of life, making it difficult to maintain employment and have successful relationships. About 30 percent of schizophrenia patients don't actually respond to antipsychotics at all. Current drugs can also have adverse side effects including elevated cholesterol, high blood pressure, diabetes and movements that patients cannot control.
The recent clinical trial heralds a new treatment approach. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” says Andrew Miller, COO of Karuna.
Scientists have been looking to develop alternatives. However, “the field of drug treatment of schizophrenia is currently in the doldrums,” says Peter McKenna, a senior researcher at FIDMAG Research Foundation in Spain which specialises in mental health.
In the 2000s there was a major push to target a brain receptor for a chemical called glutamate. Evidence suggested that this receptor is abnormal in the brains of schizophrenia patients, but attempts to try glutamate failed in clinical trials.
After that, many pharmaceutical companies dropped out of the race for a more useful treatment. But some companies continued to search, such as Karuna Therapeutics, led by founder and Chief Operating Officer Andrew Miller and CEO Steve Paul. The recent clinical trial suggests their persistence has led to an important breakthrough with their drug, KarXT. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” Miller says.
How it works
Neurotransmitters are chemical messengers that pass signals between neurons. To work effectively, neurotransmitters need a receptor to bind to. A neurotransmitter called acetylcholine seems to be especially important in schizophrenia. It interacts with sites called muscarinic receptors, which are involved in the network of nerves that calm your body after a stressful event. Post mortem studies in people with schizophrenia have shown that two muscarinic receptors in the brain, the M1 and M4 receptors, are activated at unusually low levels because they don’t receive enough signals from acetylcholine.
The M4 receptor appears to play a role in psychosis. The M1 receptor is also associated with psychosis but is primarily thought to be involved in cognition. KarXT, taken orally, works by activating both of these receptors to signal properly. It is this twofold action that seems to explain its effectiveness. “[The drug’s] design enables the preferential stimulation of these muscarinic receptors in the brain,” Miller says.
How it developed
It all started in the early 1990s when Paul was at pharmaceutical company Eli Lilly. He discovered that Xanomeline, the drug they were testing on Alzheimer's patients, had antipsychotic effects. It worked by stimulating M1 and M4 receptors, so he and his colleagues decided to test Xanomeline on schizophrenia patients, supported by research on the connection between muscarinic receptors and psychosis. They found that Xanomeline reduced both positive and negative symptoms.
Unfortunately, it also caused significant side effects. The problem was that stimulating the M1 and M4 receptors in the brain also stimulated muscarinic receptors in the body that led to severe vomiting, diarrhea and even the temporary loss of consciousness.
In the end, Eli Lilly discontinued the clinical trials for the drug, but Miller set up Karuna Therapeutics to develop a solution. “I was determined to find a way to harness the therapeutic benefit demonstrated in studies of Xanomeline, while eliminating side effects that limited its development,” Miller says.
He analysed over 7,000 possible ways of mixing Xanomeline with other agents before settling on KarXT. It combines Xanomeline with a drug called Trospium Chloride, which blocks muscarinic receptors in the body – taking care of the side effects such as vomiting – but leaves them unblocked in the brain. Paul was so excited by Miller’s progress that he joined Karuna after leaving Eli Lilly and founding two previous startups.
“It's a very important approach,” says Rick Adams, Future Leaders Fellow in the Institute of Cognitive Neuroscience and Centre for Medical Image Computing at University College London. “We are in desperate need of alternative drug targets and this target is one of the best. There are other alternative targets, but not many are as close to being successful as the muscarinic receptor drug.”
Clinical Trial
Following a successful phase 2 clinical trial in 2019, the most recent trial involved 126 patients who were given KarXT, and 126 who were given a placebo. Compared to the placebo, patients taking KarXT had a significant 9.6 point reduction in the positive and negative syndrome scale (PANSS), the standard for rating schizophrenic symptoms.
KarXT also led to statistically significant declines in positive and negative symptoms compared to the placebo. “The results suggest that KarXT could be a potentially game-changing option in the management of both positive and negative symptoms of schizophrenia,” Miller says.
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, is optimistic about the side effects but highlights the need for more safety trials.
McKenna, the researcher at FIDMAG Foundation, agrees about the drug’s potential. “The new [phase 3] study is positive,” he says. “It is reassuring that one is not dealing with a drug that works in one trial and then inexplicably fails in the next one.”
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, said the drug is an unprecedented step forward. “KarXT is one of the first drugs with a novel mechanism of action to show promise in clinical trials.”
Even though the drug blocks muscarine receptors in the body, some patients still suffered from adverse side effects like vomiting, dizziness and diarrhea. But in general, these effects were mild to moderate, especially compared to dopamine-blocking antipsychotics or Xanomeline on its own.
McCutcheon is optimistic about the side effects but highlights the need for more safety trials. “The trial results suggest that gastrointestinal side effects appear to be manageable,” he says. “We know, however, from previous antipsychotic drugs that the full picture regarding the extent of side effects can sometimes take longer to become apparent to clinicians and patients. Careful ongoing assessment during a longer period of treatment will therefore be important.”
The Future
The team is currently conducting three other trials to evaluate the efficacy and long-term safety of KarXT. Their goal is to receive FDA approval next year.
Karuna is also conducting trials to evaluate the effectiveness of KarXT in treating psychosis in patients suffering from Alzheimer’s.
The big hope is that they will soon be able to provide a radically different drug to help many patients with schizophrenia. “We are another step closer to potentially providing the first new class of medicine in more than 50 years to the millions of people worldwide living with schizophrenia,” says Miller.
Podcast: The Friday Five weekly roundup in health research
This week's Friday Five covers promising research on a potential saliva test for post-traumatic stress disorder, cancer detection with a microchip, resilient food for climate change, the best posture for pill taking, and more.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five:
- Using graphene to repair shoulders
- Testing for PTSD with saliva
- Cancer detection with a microchip
- Best posture for pill taking
- Resilient food for climate change
And an honorable mention goes to research on a new way to induce healthy fat.

