Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
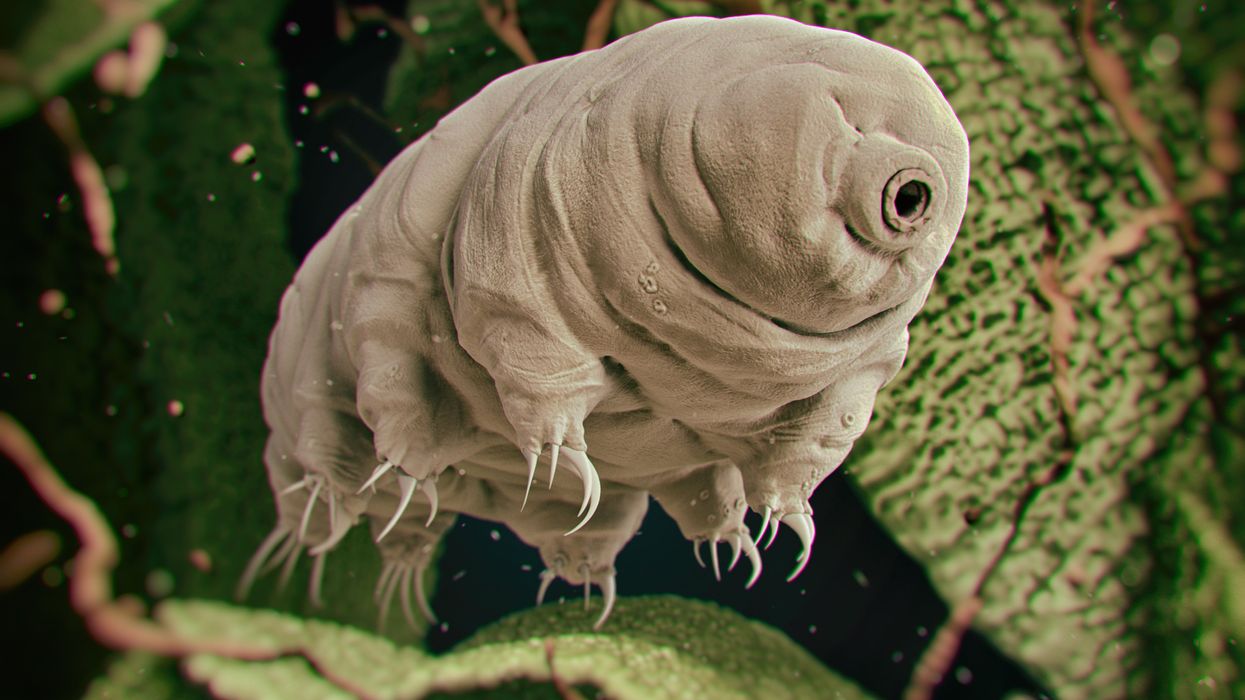
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
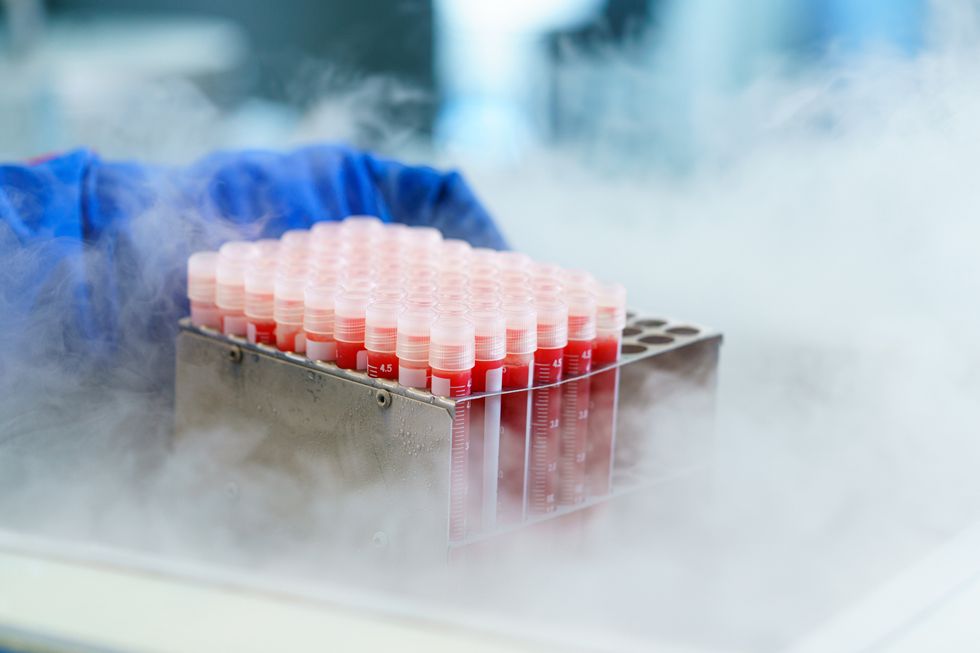
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
New study: Hotter nights, climate change, cause sleep loss with some affected more than others
According to a new study, sleep is impaired with temperatures over 50 degrees, and temps higher than 77 degrees reduce the chances of getting seven hours.
Data from the National Sleep Foundation finds that the optimal bedroom temperature for sleep is around 65 degrees Fahrenheit. But we may be getting fewer hours of "good sleepin’ weather" as the climate warms, according to a recent paper from researchers at the University of Copenhagen, Denmark.
Published in One Earth, the study finds that heat related to climate change could provide a “pathway” to sleep deprivation. The authors say the effect is “substantially larger” for those in lower-income countries. Hours of sleep decline when nighttime temperature exceeds 50 degrees, and temps higher than 77 reduce the chances of sleeping for seven hours by 3.5 percent. Even small losses associated with rising temperatures contribute significantly to people not getting enough sleep.
We’re affected by high temperatures at night because body temperature becomes more sensitive to the environment when slumbering. “Mechanisms that control for thermal regulation become more disordered during sleep,” explains Clete Kushida, a neurologist, professor of psychiatry at Stanford University and sleep medicine clinician.
The study finds that women and older adults are especially vulnerable. Worldwide, the elderly lost over twice as much sleep per degree of warming compared to younger people. This phenomenon was apparent between the ages of 60 and 70, and it increased beyond age 70. “The mechanism for balancing temperatures appears to be more affected with age,” Kushida adds.
Others disproportionately affected include those who live in regions with more greenhouse gas (GHG) emissions, which accelerate climate change, and people in hotter locales will lose more sleep per degree of warming, according to the study, with suboptimal temperatures potentially eroding 50 to 58 hours of sleep per person per year. One might think that those in warmer countries can adapt to the heat, but the researchers found no evidence for such adjustments. “We actually found those living in the warmest climate regions were impacted over twice as much as those in the coldest climate regions,” says the study's lead author, Kelton Minor, a Ph.D. candidate at the University of Copenhagen’s Center for Social Data Science.
Short sleep can reduce cognitive performance and productivity, increase absenteeism from work or school, and lead to a host of other physical and psychosocial problems. These issues include a compromised immune system, hypertension, depression, anger and suicide, say the study’s authors. According to a fact sheet by the U.S. Centers for Disease Control and Prevention, a third of U.S. adults already report sleeping fewer hours than the recommended amount, even though sufficient sleep “is not a luxury—it is something people need for good health.”
Equitable policy and planning are needed to ensure equal access to cooling technologies in a warming world.
Beyond global health, a sleep-deprived world will impact the economy as the climate warms. “Less productivity at work, associated with sleep loss or deprivation, would result in more sick days on a global scale, not just in individual countries,” Kushida says.
Unlike previous research that measured sleep patterns with self-reported surveys and controlled lab experiments, the study in One Earth offers a global analysis that relies on sleep-tracking wristbands that link more than seven million sleep records of 47,628 adults across 68 countries to local and daily meteorological data, offering new insight into the environmental impact on human sleep. Controlling for individual, seasonal and time-varying confounds, researchers found the main way that higher temperatures shorten slumber is by delaying sleep onset.
Heat effects on sleep were seen in industrialized countries including those with access to air conditioning, notes the study. Air conditioning may buffer high indoor temperatures, but they also increase GHG emissions and ambient heat displacement, thereby exacerbating the unequal burdens of global and local warming. Continued urbanization is expected to contribute to these problems.
Previous sleep studies have found an inverse U-shaped response to temperature in highly controlled settings, with subjects sleeping worse when room temperatures were either too cold or too warm. However, “people appear far better at adapting to colder outside temperatures than hotter conditions,” says Minor.
Although there are ways of countering the heat effect, some populations have more access to them. “Air conditioning can help with the effect of higher temperature, but not all individuals can afford air conditioners,” says Kushida. He points out that this could drive even greater inequity between higher- and lower-income countries.
Equitable policy and planning are needed to ensure equal access to cooling technologies in a warming world. “Clean and renewable energy systems and interventions will be needed to mitigate and adapt to ongoing climate warming,” Minor says. Future research should investigate “policy, planning and design innovation,” which could reduce the impact of sweltering temperatures on a good night’s sleep for the good of individuals, society and our planet, asserts the study.
Unabated and on its current trajectory, by 2099 suboptimal temperatures could shave 50 to 58 hours of sleep per person per year, predict the study authors. “Down the road, as technology develops, there might be ways of enabling people to adapt on a large scale to these higher temperatures,” says Kushida. “Right now, it’s not there.”
Why we need to get serious about ending aging
With the population of older people projected to grow dramatically, and the cost of healthcare with it, the future welfare of the country may depend on solving aging, writes philosopher Ingemar Patrick Linden.
It is widely acknowledged that even a small advance in anti-aging science could yield benefits in terms of healthy years that the traditional paradigm of targeting specific diseases is not likely to produce. A more youthful population would also be less vulnerable to epidemics. Approximately 93 percent of all COVID-19 deaths reported in the U.S. occurred among those aged 50 or older. The potential economic benefits would be tremendous. A more youthful population would consume less medical resources and be able to work longer. A recent study published in Nature estimates that a slowdown in aging that increases life expectancy by one year would save $38 trillion per year for the U.S. alone.
A societal effort to understand, slow down, arrest or even reverse aging of at least the size of our response to COVID-19 would therefore be a rational commitment. In fact, given that America’s older population is projected to grow dramatically, and the cost of healthcare with it, it is not an overstatement to say that the future welfare of the country may depend on solving aging.
This year, the kingdom of Saudi Arabia has announced that it will spend up to 1 billion dollars per year on science with the potential to slow down the aging process. We have also seen important investments from billionaires like Google co-founder Larry Page, Amazon founder Jeff Bezos, business magnate Larry Ellison, and PayPal co-founder Peter Thiel.
The U.S. government, however, is lagging: The National Institutes of Health spent less than one percent of its $43 billion budget for the fiscal year of 2021 on the National Institute on Aging’s Division of Aging Biology. When you visit the division’s webpage you find that their mission statement carefully omits any mention of the possibility of slowing down the aging process.
There is a lack of political will and leadership on the issue, and the idea that we should seek to do something about aging is generally met with a great deal of suspicion and trepidation. In a large representative study conducted by the Pew Research Center in 2013, only 38% of the respondents said that they would want a treatment that could slow the aging process and allow them to live at least 120 years. Apparently, most people prefer, or at least do not mind, to age and die within a natural lifespan. This result has been confirmed by smaller studies and it is, I think, surprising. Are we not supposed to live in a youth-culture? Are people not supposed to want to stay young and alive forever? Is self-preservation not the strong drive we have always assumed it to be?
We are inundated and saturated with an ideology of death-acceptance.
In my book, The Case against Death, I suggest that we have been culturally conditioned to think that it is virtuous to accept aging and death. We are taught to believe that although aging and death seem gruesome, they are what is best for us, all things considered. This is what we are supposed to think, and the majority accept it. I call this the Wise View because death acceptance has been the dominant view of philosophers since the beginning. Socrates compared our earthly life to an illness and a prison and described death as a healer and a liberator. The Buddha taught that life is suffering and that the way to escape suffering is to end the cycle of birth, death and rebirth. Stoic philosophers from Zeno to Marcus Aurelius believed that everything that happens in accordance with nature is good, and that therefore we should not only accept death but welcome it as an aspect of a perfect totality.
Epicureans agreed with these rival schools and famously argued that death cannot harm us because where we are, death is not, and where death is we are not. We cannot be harmed if we are not, so death is harmless. The simple view that death actually can harm us greatly is one of the least philosophical views one can hold.

In The Case Against Death, philosopher Ingemar Patrick Linden argues that we frown on using science to prolong healthy life only because we're culturally conditioned to think that way.
Many of the stories we tell promote the Wise View. One of the earliest known pieces of literature, the Epic of Gilgamesh, follows Gilgamesh on a quest for eternal life ending with the wisdom that death is the destiny of man. Today we learn about the tedium of immortality from the children’s book Tuck Everlasting by Natalie Babbitt, and we are warned about the vice of wanting to resist death in other books and films such as J.K Rowling’s Harry Potter, where Voldemort must kill Harry as a step towards his own immortality; C.S. Lewis’ The Chronicles of Narnia where the White Witch has gained immortal youth and madness in equal measures; J.R.R. Tolkien’s Lord of the Rings trilogy where the ring extends the wearer’s life but can also destroy them, as exemplified by the creep Gollum; and Doctor Strange where life extension is the one magical power that is taboo. In Star Wars, Yoda, a stereotype of the sage, teaches us the wisdom handed down by philosophers and prophets: “Death is a natural part of life. Rejoice for those around you who transform into the Force. Mourn them do not. Miss them do not.”
We are inundated and saturated with an ideology of death-acceptance. Can the dear reader name one single story where the hero is pursuing anti-aging, longevity or immortality and the villain tries to stop her?
The Wise View resonates with us partly because we think that there is nothing we can do about aging and death, so we do not want to wish for what we cannot have. Youth and immortality are sour grapes to us. Believing that death is, all things considered, not such a bad thing, protects us from experiencing our aging and approaching death as a gruesome tragedy. This need to escape the thought that we are heading towards a personal catastrophe explains why many are so quick to accept arguments against radical life extension, despite their often glaring weaknesses.
One of the most common objections to radical life extension is that aging and death are natural. The problem with this argument is that many things that are natural are very bad, such as cancer, and other things that are not natural are very good, such as a cure for cancer. Why are we so sure that cancer is bad? Because we assume that it is bad to die. Indeed, nothing is more natural than wanting to live. We seem to need philosophers and story tellers to talk us out of it and, in the words of a distinguished bioethicist, “instruct and somewhat moderate our lust for life.”
Another standard objection is that we need a deadline, and that without death we could postpone every action forever. “Death brings urgency and seriousness to life,” say proponents of this view, but there are several problems with this argument. Even if our lives were endless, there would still be many things we would have to do at a certain time, and that could not be redone, for example, saving our planet from being destroyed, or becoming the first person on Venus. And if we prefer pleasant endless lives over unpleasant endless ones, we will have to exercise, eat right, keep our word, develop our talents, show up for time at work, pay our taxes by the due date, remember birthdays, and so on.
The Wise View provides us with a feel-good bromide for the anxiety created by the foreknowledge of our decay and death by telling us that these are not evils, but blessings in disguise. Once perhaps an innocuous delusion, today the view stands in the way of a necessary societal commitment to research that can prolong our healthy life.
Besides, even if we succeeded in ending aging, we would still die from other causes. Given the rate of accidental deaths we would be fortunate to live to age 2000 all things equal. So even if, contrary to what I have argued, we do need a deadline, we can still argue that the natural lifespan that we now labor under is inhuman and that it forces each human to limit her ambitions and to become only a fragment of all that she that could have been. Our tight time constraint imposes tragic choices and inflated opportunity costs. Death does not make life matter; it makes time matter.
The perhaps most awful argument against radical life extension is grounded in a pessimism that holds life in such little regard that it says that best of all is never to have born. This view was expressed by Ecclesiastes in the Hebrew Bible, by Sophocles and several other ancient Greeks, by the German philosopher Arthur Schopenhauer, and recently by, among others, the South African philosopher David Benatar who argues that it is wrong to bring children into the world and that we should euthanize all sentient life. Pessimism, one suspects, largely appeals to some for reasons having to do with personal temperament, but insofar as it is built on factual beliefs, they can be addressed by providing a less negatively biased understanding of the world, by pointing out that curing aging would decrease the badness that they are so hypersensitive to, and by reminding them that if life really becomes unbearable, they are free to quit at any time. Other means of persuasion could include recommending sleep, exercise and taking long brisk walks in nature.
The Wise View provides us with a feel-good bromide for the anxiety created by the foreknowledge of our decay and death by telling us that these are not evils, but blessings in disguise. Once perhaps an innocuous delusion, today the view stands in the way of a necessary societal commitment to research that can prolong our healthy life. We need abandon it and openly admit that aging is a scourge that deserves to be fought with the combined energies equaling those expended on fighting COVID-19, Alzheimer’s disease, cancer, stroke and all the other illnesses for which aging is the greatest risk factor. The fight to end aging transcends ordinary political boundaries and is therefore the kind of grand unifying enterprise that could re-energize a society suffering from divisiveness and the sense of a lack of a common purpose. It is hard to imagine a more worthwhile cause.

