The Algorithm Will See You Now

Artificial intelligence in medicine, still in an early phase, stands to transform how doctors and nurses spend their time.
There's a quiet revolution going on in medicine. It's driven by artificial intelligence, but paradoxically, new technology may put a more human face on healthcare.
AI's usefulness in healthcare ranges far and wide.
Artificial intelligence is software that can process massive amounts of information and learn over time, arriving at decisions with striking accuracy and efficiency. It offers greater accuracy in diagnosis, exponentially faster genome sequencing, the mining of medical literature and patient records at breathtaking speed, a dramatic reduction in administrative bureaucracy, personalized medicine, and even the democratization of healthcare.
The algorithms that bring these advantages won't replace doctors; rather, by offloading some of the most time-consuming tasks in healthcare, providers will be able to focus on personal interactions with patients—listening, empathizing, educating and generally putting the care back in healthcare. The relationship can focus on the alleviation of suffering, both the physical and emotional kind.
Challenges of Getting AI Up and Running
The AI revolution, still in its early phase in medicine, is already spurring some amazing advances, despite the fact that some experts say it has been overhyped. IBM's Watson Health program is a case in point. IBM capitalized on Watson's ability to process natural language by designing algorithms that devour data like medical articles and analyze images like MRIs and medical slides. The algorithms help diagnose diseases and recommend treatment strategies.
But Technology Review reported that a heavily hyped partnership with the MD Anderson Cancer Center in Houston fell apart in 2017 because of a lack of data in the proper format. The data existed, just not in a way that the voraciously data-hungry AI could use to train itself.
The hiccup certainly hasn't dampened the enthusiasm for medical AI among other tech giants, including Google and Apple, both of which have invested billions in their own healthcare projects. At this point, the main challenge is the need for algorithms to interpret a huge diversity of data mined from medical records. This can include everything from CT scans, MRIs, electrocardiograms, x-rays, and medical slides, to millions of pages of medical literature, physician's notes, and patient histories. It can even include data from implantables and wearables such as the Apple Watch and blood sugar monitors.
None of this information is in anything resembling a standard format across and even within hospitals, clinics, and diagnostic centers. Once the algorithms are trained, however, they can crunch massive amounts of data at blinding speed, with an accuracy that matches and sometimes even exceeds that of highly experienced doctors.
Genome sequencing, for example, took years to accomplish as recently as the early 2000s. The Human Genome Project, the first sequencing of the human genome, was an international effort that took 13 years to complete. In April of this year, Rady Children's Institute for Genomic Medicine in San Diego used an AI-powered genome sequencing algorithm to diagnose rare genetic diseases in infants in about 20 hours, according to ScienceDaily.
"Patient care will always begin and end with the doctor."
Dr. Stephen Kingsmore, the lead author of an article published in Science Translational Medicine, emphasized that even though the algorithm helped guide the treatment strategies of neonatal intensive care physicians, the doctor was still an indispensable link in the chain. "Some people call this artificial intelligence, we call it augmented intelligence," he says. "Patient care will always begin and end with the doctor."
One existing trend is helping to supply a great amount of valuable data to algorithms—the electronic health record. Initially blamed for exacerbating the already crushing workload of many physicians, the EHR is emerging as a boon for algorithms because it consolidates all of a patient's data in one record.
Examples of AI in Action Around the Globe
If you're a parent who has ever taken a child to the doctor with flulike symptoms, you know the anxiety of wondering if the symptoms signal something serious. Kang Zhang, M.D., Ph.D., the founding director of the Institute for Genomic Medicine at the University of California at San Diego, and colleagues developed an AI natural language processing model that used deep learning to analyze the EHRs of 1.3 million pediatric visits to a clinic in Guanzhou, China.
The AI identified common childhood diseases with about the same accuracy as human doctors, and it was even able to split the diagnoses into two categories—common conditions such as flu, and serious, life-threatening conditions like meningitis. Zhang has emphasized that the algorithm didn't replace the human doctor, but it did streamline the diagnostic process and could be used in a triage capacity when emergency room personnel need to prioritize the seriously ill over those suffering from common, less dangerous ailments.
AI's usefulness in healthcare ranges far and wide. In Uganda and several other African nations, AI is bringing modern diagnostics to remote villages that have no access to traditional technologies such as x-rays. The New York Times recently reported that there, doctors are using a pocket-sized, hand-held ultrasound machine that works in concert with a cell phone to image and diagnose everything from pneumonia (a common killer of children) to cancerous tumors.
The beauty of the highly portable, battery-powered device is that ultrasound images can be uploaded on computers so that physicians anywhere in the world can review them and weigh in with their advice. And the images are instantly incorporated into the patient's EHR.
Jonathan Rothberg, the founder of Butterfly Network, the Connecticut company that makes the device, told The New York Times that "Two thirds of the world's population gets no imaging at all. When you put something on a chip, the price goes down and you democratize it." The Butterfly ultrasound machine, which sells for $2,000, promises to be a game-changer in remote areas of Africa, South America, and Asia, as well as at the bedsides of patients in developed countries.
AI algorithms are rapidly emerging in healthcare across the U.S. and the world. China has become a major international player, set to surpass the U.S. this year in AI capital investment, the translation of AI research into marketable products, and even the number of often-cited research papers on AI. So far the U.S. is still the leader, but some experts describe the relationship between the U.S. and China as an AI cold war.
"The future of machine learning isn't sentient killer robots. It's longer human lives."
The U.S. Food and Drug Administration expanded its approval of medical algorithms from two in all of 2017 to about two per month throughout 2018. One of the first fields to be impacted is ophthalmology.
One algorithm, developed by the British AI company DeepMind (owned by Alphabet, the parent company of Google), instantly scans patients' retinas and is able to diagnose diabetic retinopathy without needing an ophthalmologist to interpret the scans. This means diabetics can get the test every year from their family physician without having to see a specialist. The Financial Times reported in March that the technology is now being used in clinics throughout Europe.
In Copenhagen, emergency service dispatchers are using a new voice-processing AI called Corti to analyze the conversations in emergency phone calls. The algorithm analyzes the verbal cues of callers, searches its huge database of medical information, and provides dispatchers with onscreen diagnostic information. Freddy Lippert, the CEO of EMS Copenhagen, notes that the algorithm has already saved lives by expediting accurate diagnoses in high-pressure situations where time is of the essence.
Researchers at the University of Nottingham in the UK have even developed a deep learning algorithm that predicts death more accurately than human clinicians. The algorithm incorporates data from a huge range of factors in a chronically ill population, including how many fruits and vegetables a patient eats on a daily basis. Dr. Stephen Weng, lead author of the study, published in PLOS ONE, said in a press release, "We found machine learning algorithms were significantly more accurate in predicting death than the standard prediction models developed by a human expert."
New digital technologies are allowing patients to participate in their healthcare as never before. A feature of the new Apple Watch is an app that detects cardiac arrhythmias and even produces an electrocardiogram if an abnormality is detected. The technology, approved by the FDA, is helping cardiologists monitor heart patients and design interventions for those who may be at higher risk of a cardiac event like a stroke.
If having an algorithm predict your death sends a shiver down your spine, consider that algorithms may keep you alive longer. In 2018, technology reporter Tristan Greene wrote for Medium that "…despite the unending deluge of panic-ridden articles declaring AI the path to apocalypse, we're now living in a world where algorithms save lives every day. The future of machine learning isn't sentient killer robots. It's longer human lives."
The Risks of AI Compiling Your Data
To be sure, the advent of AI-infused medical technology is not without its risks. One risk is that the use of AI wearables constantly monitoring our vital signs could turn us into a nation of hypochondriacs, racing to our doctors every time there's a blip in some vital sign. Such a development could stress an already overburdened system that suffers from, among other things, a shortage of doctors and nurses. Another risk has to do with the privacy protections on the massive repository of intimately personal information that AI will have on us.
In an article recently published in the Journal of the American Medical Association, Australian researcher Kit Huckvale and colleagues examined the handling of data by 36 smartphone apps that assisted people with either depression or smoking cessation, two areas that could lend themselves to stigmatization if they fell into the wrong hands.
Out of the 36 apps, 33 shared their data with third parties, despite the fact that just 25 of those apps had a privacy policy at all and out of those, only 23 stated that data would be shared with third parties. The recipients of all that data? It went almost exclusively to Facebook and Google, to be used for advertising and marketing purposes. But there's nothing to stop it from ending up in the hands of insurers, background databases, or any other entity.
Even when data isn't voluntarily shared, any digital information can be hacked. EHRs and even wearable devices share the same vulnerability as any other digital record or device. Still, the promise of AI to radically improve efficiency and accuracy in healthcare is hard to ignore.
AI Can Help Restore Humanity to Medicine
Eric Topol, director of the Scripps Research Translational Institute and author of the new book Deep Medicine, says that AI gives doctors and nurses the most precious gift of all: time.
Topol welcomes his patients' use of the Apple Watch cardiac feature and is optimistic about the ways that AI is revolutionizing medicine. He says that the watch helps doctors monitor how well medications are working and has already helped to prevent strokes. But in addition to that, AI will help bring the humanity back to a profession that has become as cold and hard as a stainless steel dissection table.
"When I graduated from medical school in the 1970s," he says, "you had a really intimate relationship with your doctor." Over the decades, he has seen that relationship steadily erode as medical organizations demanded that doctors see more and more patients within ever-shrinking time windows.
"Doctors have no time to think, to communicate. We need to restore the mission in medicine."
In addition to that, EHRs have meant that doctors and nurses are getting buried in paperwork and administrative tasks. This is no doubt one reason why a recent study by the World Health Organization showed that worldwide, about 50 percent of doctors suffer from burnout. People who are utterly exhausted make more mistakes, and medical clinicians are no different from the rest of us. Only medical mistakes have unacceptably high stakes. According to its website, Johns Hopkins University recently announced that in the U.S. alone, 250,000 people die from medical mistakes each year.
"Doctors have no time to think, to communicate," says Topol. "We need to restore the mission in medicine." AI is giving doctors more time to devote to the thing that attracted them to medicine in the first place—connecting deeply with patients.
There is a real danger at this juncture, though, that administrators aware of the time-saving aspects of AI will simply push doctors to see more patients, read more tests, and embrace an even more crushing workload.
"We can't leave it to the administrators to just make things worse," says Topol. "Now is the time for doctors to advocate for a restoration of the human touch. We need to stand up for patients and for the patient-doctor relationship."
AI could indeed be a game changer, he says, but rather than squander the huge benefits of more time, "We need a new equation going forward."
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
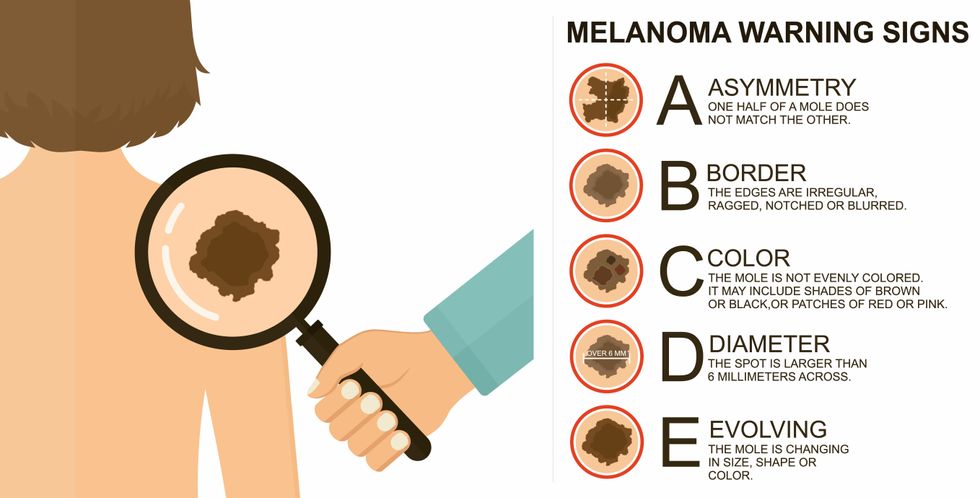
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

