The Dangers of Hype: How a Bold Claim and Sensational Media Unraveled a Company
Magnetic resonance imaging of the brain.
This past March, headlines suddenly flooded the Internet about a startup company called Nectome. Founded by two graduates of the Massachusetts Institute of Technology, the new company was charging people $10,000 to join a waiting list to have their brains embalmed, down to the last neuron, using an award-winning chemical compound.
While the lay public presumably burnt their wills and grew ever more excited about the end of humanity's quest for immortality, neurologists let out a collective sigh.
Essentially, participants' brains would turn to a substance like glass and remain in a state of near-perfect preservation indefinitely. "If memories can truly be preserved by a sufficiently good brain banking technique," Nectome's website explains, "we believe that within the century it could become feasible to digitize your preserved brain and use that information to recreate your mind." But as with most Faustian bargains, Nectome's proposition came with a serious caveat -- death.
That's right, in order for Nectome's process to properly preserve your connectome, the comprehensive map of the brain's neural connections, you must be alive (and under anesthesia) while the fluid is injected. This way, the company postulates, when the science advances enough to read and extract your memories someday, your vitrified brain will still contain your perfectly preserved essence--which can then be digitally recreated as a computer simulation.
Almost immediately this story gained buzz with punchy headlines: "Startup wants to upload your brain to the cloud, but has to kill you to do it," "San Junipero is real: Nectome wants to upload your brain," and "New tech firm promises eternal life, but you have to die."
While the lay public presumably burnt their wills and grew ever more excited about the end of humanity's quest for immortality, neurologists let out a collective sigh -- hype had struck the scientific community once again.
The truth about Nectome is that its claims are highly speculative and no hard science exists to suggest that our connectome is the key to our 'being,' nor that it can ever be digitally revived. "We haven't come even close to understanding even the most basic types of functioning in the brain," says neuroscientist Alex Fox, who was educated at the University of Queensland in Australia. "Memory storage in the brain is only a theoretical concept [and] there are some seriously huge gaps in our knowledge base that stand in the way of testing [the connectome] theory."
After the Nectome story broke, Harvard computational neuroscientist Sam Gershman tweeted out:
"Didn't anyone tell them that we've known the C Elegans (a microscopic worm) connectome for over a decade but haven't figured out how to reconstruct all of their memories? And that's only 7000 synapses compared to the trillions of synapses in the human brain!"
Hype can come from researchers themselves, who are under an enormous amount of pressure to publish original work and maintain funding.
How media coverage of Nectome went from an initial fastidiously researched article in the MIT Technology Review by veteran science journalist Antonio Regalado to the click-bait frenzy it became is a prime example of the 'science hype' phenomenon. According to Adam Auch, who holds a doctorate in philosophy from Dalhousie University in Nova Scotia, Canada, "Hype is a feature of all stages of the scientific dissemination process, from the initial circulation of preliminary findings within particular communities of scientists, to the process by which such findings come to be published in peer-reviewed journals, to the subsequent uptake these findings receive from the non-specialist press and the general public."
In the case of Nectome, hype was present from the word go. Riding the high of several major wins, including having raised over one million dollars in funding and partnering with well-known MIT neurologist Edward Boyden, Nectome founders Michael McCanna and Robert McIntyre launched their website on March 1, 2018. Just one month prior, they were able to purchase and preserve a newly deceased corpse in Portland, Oregon, showing that vitrifixation, their method of chemical preservation, could be used on a human specimen. It had previously won an award for preserving every synaptic structure on a rabbit brain.
The Nectome mission statement, found on its website, is laced with saccharine language that skirts the unproven nature of the procedure the company is peddling for big bucks: "Our mission is to preserve your brain well enough to keep all its memories intact: from that great chapter of your favorite book to the feeling of cold winter air, baking an apple pie, or having dinner with your friends and family."
This rhetoric is an example of hype that can come from researchers themselves, who are under an enormous amount of pressure to publish original work and maintain funding. As a result, there is a constant push to present science as "groundbreaking" when really, as is apparently the case with Nectome, it is only a small piece in a much larger effort.
Calling out the audacity of Nectome's posited future, neuroscientist Gershman commented to another publication, "The important question is whether the connectome is sufficient for memory: Can I reconstruct all memories knowing only the connections between neurons? The answer is almost certainly no, given our knowledge about how memories are stored (itself a controversial topic)."

The former home page of Nectome's website, which has now been replaced by a statement titled, "Response to recent press."
Furthermore, universities like MIT, who entered into a subcontract with Nectome, are under pressure to seek funding through partnerships with industry as a result of the Bayh-Dole Act of 1980. Also known as the Patent and Trademark Law Amendments Act, this piece of legislation allows universities to commercialize inventions developed under federally funded research programs, like Nectome's method of preserving brains, formally called Aldehyde-Stabilized Cryopreservation.
"[Universities use] every incentive now to talk about innovation," explains Dr. Ivan Oransky, president of the Association of Health Care Journalists and co-founder of retractionwatch.com, a blog that catalogues errors and fraud in published research. "Innovation to me is often a fancy word for hype. The role of journalists should not be to glorify what universities [say, but to] tell the closest version of the truth they can."
In this case, a combination of the hyperbolic press, combined with some impressively researched expose pieces, led MIT to cut its ties with Nectome on April 2nd, 2018, just two weeks after the news of their company broke.
The solution to the dangers of hype, experts say, is a more scientifically literate public—and less clickbait-driven journalism.
Because of its multi-layered nature, science hype carries several disturbing consequences. For one, exaggerated coverage of a discovery could mislead the public by giving them false hope or unfounded worry. And media hype can contribute to a general mistrust of science. In these instances, people might, as Auch puts it, "fall back on previously held beliefs, evocative narratives, or comforting biases instead of well-justified scientific evidence."
All of this is especially dangerous in today's 'fake news' era, when companies or political parties sow public confusion for their own benefit, such as with global warming. In the case of Nectome, the danger is that people might opt to end their lives based off a lacking scientific theory. In fact, the company is hoping to enlist terminal patients in California, where doctor-assisted suicide is legal. And 25 people have paid the $10,000 to join Nectome's waiting list, including Sam Altman, president of the famed startup accelerator Y Combinator. Nectome now has offered to refund the money.
Founders McCanna and McIntyre did not return repeated requests for comment for this article. A new statement on their website begins: "Vitrifixation today is a powerful research tool, but needs more research and development before anyone considers applying it in a context other than research."
The solution to the dangers of hype, experts say, is a more scientifically literate public—and less clickbait-driven journalism. Until then, it seems that companies like Nectome will continue to enjoy at least 15 minutes of fame.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
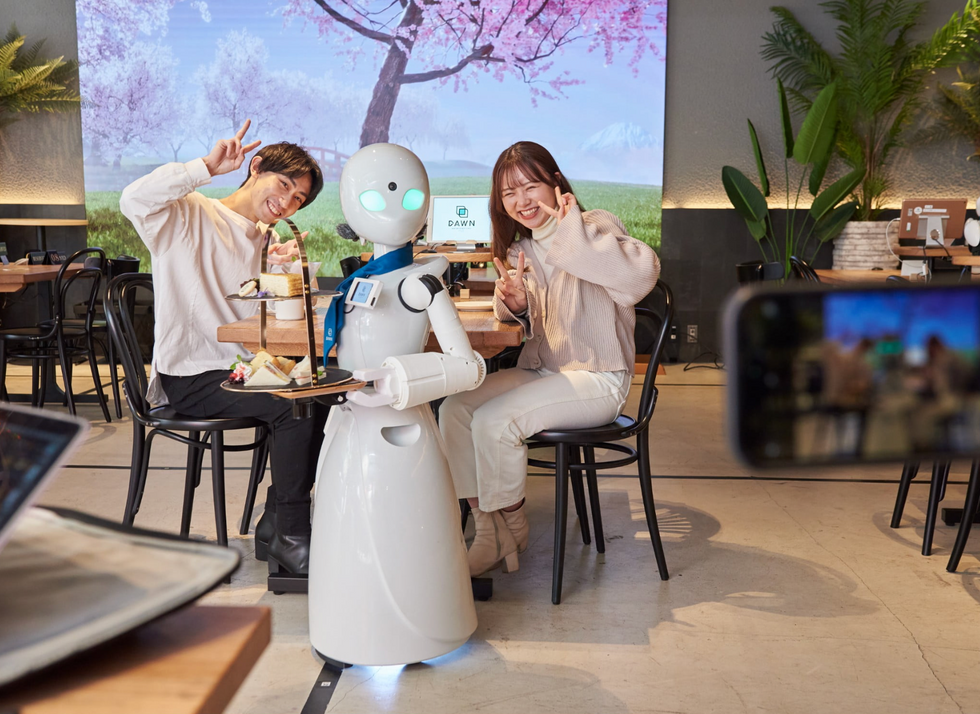
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

