The Shiny–and Potentially Dangerous—New Tool for Predicting Human Behavior

Studies of twins have played an important role in determining that genetic differences play a role in the development of differences in behavior.
[Editor's Note: This essay is in response to our current Big Question, which we posed to experts with different perspectives: "How should DNA tests for intelligence be used, if at all, by parents and educators?"]
Imagine a world in which pregnant women could go to the doctor and obtain a simple inexpensive genetic test of their unborn child that would allow them to predict how tall he or she would eventually be. The test might also tell them the child's risk for high blood pressure or heart disease.
Can we use DNA not to understand, but to predict who is going to be intelligent or extraverted or mentally ill?
Even more remarkable -- and more dangerous -- the test might predict how intelligent the child would be, or how far he or she could be expected to go in school. Or heading further out, it might predict whether he or she will be an alcoholic or a teetotaler, or straight or gay, or… you get the idea. Is this really possible? If it is, would it be a good idea? Answering these questions requires some background in a scientific field called behavior genetics.
Differences in human behavior -- intelligence, personality, mental illness, pretty much everything -- are related to genetic differences among people. Scientists have known this for 150 years, ever since Darwin's half-cousin Francis Galton first applied Shakespeare's phrase, "Nature and Nurture" to the scientific investigation of human differences. We knew about the heritability of behavior before Mendel's laws of genetics had been re-discovered at the end of the last century, and long before the structure of DNA was discovered in the 1950s. How could discoveries about genetics be made before a science of genetics even existed?
The answer is that scientists developed clever research designs that allowed them to make inferences about genetics in the absence of biological knowledge about DNA. The best-known is the twin study: identical twins are essentially clones, sharing 100 percent of their DNA, while fraternal twins are essentially siblings, sharing half. To the extent that identical twins are more similar for some trait than fraternal twins, one can infer that heredity is playing a role. Adoption studies are even more straightforward. Is the personality of an adopted child more like the biological parents she has never seen, or the adoptive parents who raised her?
Twin and adoption studies played an important role in establishing beyond any reasonable doubt that genetic differences play a role in the development of differences in behavior, but they told us very little about how the genetics of behavior actually worked. When the human genome was finally sequenced in the early 2000s, and it became easier and cheaper to obtain actual DNA from large samples of people, scientists anticipated that we would soon find the genes for intelligence, mental illness, and all the other behaviors that were known to be "heritable" in a general way.
But to everyone's amazement, the genes weren't there. It turned out that there are thousands of genes related to any given behavior, so many that they can't be counted, and each one of them has such a tiny effect that it can't be tied to meaningful biological processes. The whole scientific enterprise of understanding the genetics of behavior seemed ready to collapse, until it was rescued -- sort of -- by a new method called polygenic scores, PGS for short. Polygenic scores abandon the old task of finding the genes for complex human behavior, replacing it with black-box prediction: can we use DNA not to understand, but to predict who is going to be intelligent or extraverted or mentally ill?
Prediction from observing parents works better, and is far easier and cheaper, than anything we can do with DNA.
PGS are the shiny new toy of human genetics. From a technological standpoint they are truly amazing, and they are useful for some scientific applications that don't involve making decisions about individual people. We can obtain DNA from thousands of people, estimate the tiny relationships between individual bits of DNA and any outcome we want — height or weight or cardiac disease or IQ — and then add all those tiny effects together into a single bell-shaped score that can predict the outcome of interest. In theory, we could do this from the moment of conception.
Polygenic scores for height already work pretty well. Physicians are debating whether the PGS for heart disease are robust enough to be used in the clinic. For some behavioral traits-- the most data exist for educational attainment -- they work well enough to be scientifically interesting, if not practically useful. For traits like personality or sexual orientation, the prediction is statistically significant but nowhere close to practically meaningful. No one knows how much better any of these predictions are likely to get.
Without a doubt, PGS are an amazing feat of genomic technology, but the task they accomplish is something scientists have been able to do for a long time, and in fact it is something that our grandparents could have done pretty well. PGS are basically a new way to predict a trait in an individual by using the same trait in the individual's parents — a way of observing that the acorn doesn't fall far from the tree.
The children of tall people tend to be tall. Children of excellent athletes are athletic; children of smart people are smart; children of people with heart disease are at risk, themselves. Not every time, of course, but that is how imperfect prediction works: children of tall parents vary in their height like anyone else, but on average they are taller than the rest of us. Prediction from observing parents works better, and is far easier and cheaper, than anything we can do with DNA.
But wait a minute. Prediction from parents isn't strictly genetic. Smart parents not only pass on their genes to their kids, but they also raise them. Smart families are privileged in thousands of ways — they make more money and can send their kids to better schools. The same is true for PGS.
The ability of a genetic score to predict educational attainment depends not only on examining the relationship between certain genes and how far people go in school, but also on every personal and social characteristic that helps or hinders education: wealth, status, discrimination, you name it. The bottom line is that for any kind of prediction of human behavior, separation of genetic from environmental prediction is very difficult; ultimately it isn't possible.
Still, experts are already discussing how to use PGS to make predictions for children, and even for embryos.
This is a reminder that we really have no idea why either parents or PGS predict as well or as poorly as they do. It is easy to imagine that a PGS for educational attainment works because it is summarizing genes that code for efficient neurological development, bigger brains, and swifter problem solving, but we really don't know that. PGS could work because they are associated with being rich, or being motivated, or having light skin. It's the same for predicting from parents. We just don't know.
Still, experts are already discussing how to use PGS to make predictions for children, and even for embryos.
For example, maybe couples could fertilize multiple embryos in vitro, test their DNA, and select the one with the "best" PGS on some trait. This would be a bad idea for a lot of reasons. Such scores aren't effective enough to be very useful to parents, and to the extent they are effective, it is very difficult to know what other traits might be selected for when parents try to prioritize intelligence or attractiveness. People will no doubt try it anyway, and as a matter of reproductive freedom I can't think of any way to stop them. Fortunately, the practice probably won't have any great impact one way or another.
That brings us to the ethics of PGS, particularly in the schools. Imagine that when a child enrolls in a public school, an IQ test is given to her biological parents. Children with low-IQ parents are statistically more likely to have low IQs themselves, so they could be assigned to less demanding classrooms or vocational programs. Hopefully we agree that this would be unethical, but let's think through why.
First of all, it would be unethical because we don't know why the parents have low IQs, or why their IQs predict their children's. The parents could be from a marginalized ethnic group, recognizable by their skin color and passed on genetically to their children, so discriminating based on a parent's IQ would just be a proxy for discriminating based on skin color. Such a system would be no more than a social scientific gloss on an old-fashioned program for perpetuating economic and cognitive privilege via the educational system.
People deserve to be judged on the basis of their own behavior, not a genetic test.
Assigning children to classrooms based on genetic testing would be no different, although it would have the slight ethical advantage of being less effective. The PGS for educational attainment could reflect brain-efficiency, but it could also depend on skin color, or economic advantage, or personality, or literally anything that is related in any way to economic success. Privileging kids with higher genetic scores would be no different than privileging children with smart parents. If schools really believe that a psychological trait like IQ is important for school placement, the sensible thing is to administer the children an actual IQ test – not a genetic test.
IQ testing has its own issues, of course, but at least it involves making decisions about individuals based on their own observable characteristics, rather than on characteristics of their parents or their genome. If decisions must be made, if resources must be apportioned, people deserve to be judged on the basis of their own behavior, the content of their character. Since it can't be denied that people differ in all sorts of relevant ways, this is what it means for all people to be created equal.
[Editor's Note: Read another perspective in the series here.]
Nobel Prize goes to technology for mRNA vaccines
Katalin Karikó, pictured, and Drew Weissman won the Nobel Prize for advances in mRNA research that led to the first Covid vaccines.
When Drew Weissman received a call from Katalin Karikó in the early morning hours this past Monday, he assumed his longtime research partner was calling to share a nascent, nagging idea. Weissman, a professor of medicine at the Perelman School of Medicine at the University of Pennsylvania, and Karikó, a professor at Szeged University and an adjunct professor at UPenn, both struggle with sleep disturbances. Thus, middle-of-the-night discourses between the two, often over email, has been a staple of their friendship. But this time, Karikó had something more pressing and exciting to share: They had won the 2023 Nobel Prize in Physiology or Medicine.
The work for which they garnered the illustrious award and its accompanying $1,000,000 cash windfall was completed about two decades ago, wrought through long hours in the lab over many arduous years. But humanity collectively benefited from its life-saving outcome three years ago, when both Moderna and Pfizer/BioNTech’s mRNA vaccines against COVID were found to be safe and highly effective at preventing severe disease. Billions of doses have since been given out to protect humans from the upstart viral scourge.
“I thought of going somewhere else, or doing something else,” said Katalin Karikó. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
Unlocking the power of mRNA
Weissman and Karikó unlocked mRNA vaccines for the world back in the early 2000s when they made a key breakthrough. Messenger RNA molecules are essentially instructions for cells’ ribosomes to make specific proteins, so in the 1980s and 1990s, researchers started wondering if sneaking mRNA into the body could trigger cells to manufacture antibodies, enzymes, or growth agents for protecting against infection, treating disease, or repairing tissues. But there was a big problem: injecting this synthetic mRNA triggered a dangerous, inflammatory immune response resulting in the mRNA’s destruction.
While most other researchers chose not to tackle this perplexing problem to instead pursue more lucrative and publishable exploits, Karikó stuck with it. The choice sent her academic career into depressing doldrums. Nobody would fund her work, publications dried up, and after six years as an assistant professor at the University of Pennsylvania, Karikó got demoted. She was going backward.
“I thought of going somewhere else, or doing something else,” Karikó told Stat in 2020. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
A tale of tenacity
Collaborating with Drew Weissman, a new professor at the University of Pennsylvania, in the late 1990s helped provide Karikó with the tenacity to continue. Weissman nurtured a goal of developing a vaccine against HIV-1, and saw mRNA as a potential way to do it.
“For the 20 years that we’ve worked together before anybody knew what RNA is, or cared, it was the two of us literally side by side at a bench working together,” Weissman said in an interview with Adam Smith of the Nobel Foundation.
In 2005, the duo made their 2023 Nobel Prize-winning breakthrough, detailing it in a relatively small journal, Immunity. (Their paper was rejected by larger journals, including Science and Nature.) They figured out that chemically modifying the nucleoside bases that make up mRNA allowed the molecule to slip past the body’s immune defenses. Karikó and Weissman followed up that finding by creating mRNA that’s more efficiently translated within cells, greatly boosting protein production. In 2020, scientists at Moderna and BioNTech (where Karikó worked from 2013 to 2022) rushed to craft vaccines against COVID, putting their methods to life-saving use.
The future of vaccines
Buoyed by the resounding success of mRNA vaccines, scientists are now hurriedly researching ways to use mRNA medicine against other infectious diseases, cancer, and genetic disorders. The now ubiquitous efforts stand in stark contrast to Karikó and Weissman’s previously unheralded struggles years ago as they doggedly worked to realize a shared dream that so many others shied away from. Katalin Karikó and Drew Weissman were brave enough to walk a scientific path that very well could have ended in a dead end, and for that, they absolutely deserve their 2023 Nobel Prize.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Scientists turn pee into power in Uganda
With conventional fuel cells as their model, researchers learned to use similar chemical reactions to make a fuel from microbes in pee.
At the edge of a dirt road flanked by trees and green mountains outside the town of Kisoro, Uganda, sits the concrete building that houses Sesame Girls School, where girls aged 11 to 19 can live, learn and, at least for a while, safely use a toilet. In many developing regions, toileting at night is especially dangerous for children. Without electrical power for lighting, kids may fall into the deep pits of the latrines through broken or unsteady floorboards. Girls are sometimes assaulted by men who hide in the dark.
For the Sesame School girls, though, bright LED lights, connected to tiny gadgets, chased the fears away. They got to use new, clean toilets lit by the power of their own pee. Some girls even used the light provided by the latrines to study.
Urine, whether animal or human, is more than waste. It’s a cheap and abundant resource. Each day across the globe, 8.1 billion humans make 4 billion gallons of pee. Cows, pigs, deer, elephants and other animals add more. By spending money to get rid of it, we waste a renewable resource that can serve more than one purpose. Microorganisms that feed on nutrients in urine can be used in a microbial fuel cell that generates electricity – or "pee power," as the Sesame girls called it.
Plus, urine contains water, phosphorus, potassium and nitrogen, the key ingredients plants need to grow and survive. Human urine could replace about 25 percent of current nitrogen and phosphorous fertilizers worldwide and could save water for gardens and crops. The average U.S. resident flushes a toilet bowl containing only pee and paper about six to seven times a day, which adds up to about 3,500 gallons of water down per year. Plus cows in the U.S. produce 231 gallons of the stuff each year.
Pee power
A conventional fuel cell uses chemical reactions to produce energy, as electrons move from one electrode to another to power a lightbulb or phone. Ioannis Ieropoulos, a professor and chair of Environmental Engineering at the University of Southampton in England, realized the same type of reaction could be used to make a fuel from microbes in pee.
Bacterial species like Shewanella oneidensis and Pseudomonas aeruginosa can consume carbon and other nutrients in urine and pop out electrons as a result of their digestion. In a microbial fuel cell, one electrode is covered in microbes, immersed in urine and kept away from oxygen. Another electrode is in contact with oxygen. When the microbes feed on nutrients, they produce the electrons that flow through the circuit from one electrod to another to combine with oxygen on the other side. As long as the microbes have fresh pee to chomp on, electrons keep flowing. And after the microbes are done with the pee, it can be used as fertilizer.
These microbes are easily found in wastewater treatment plants, ponds, lakes, rivers or soil. Keeping them alive is the easy part, says Ieropoulos. Once the cells start producing stable power, his group sequences the microbes and keeps using them.
Like many promising technologies, scaling these devices for mass consumption won’t be easy, says Kevin Orner, a civil engineering professor at West Virginia University. But it’s moving in the right direction. Ieropoulos’s device has shrunk from the size of about three packs of cards to a large glue stick. It looks and works much like a AAA battery and produce about the same power. By itself, the device can barely power a light bulb, but when stacked together, they can do much more—just like photovoltaic cells in solar panels. His lab has produced 1760 fuel cells stacked together, and with manufacturing support, there’s no theoretical ceiling, he says.
Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofit into urban wastewater utilities.
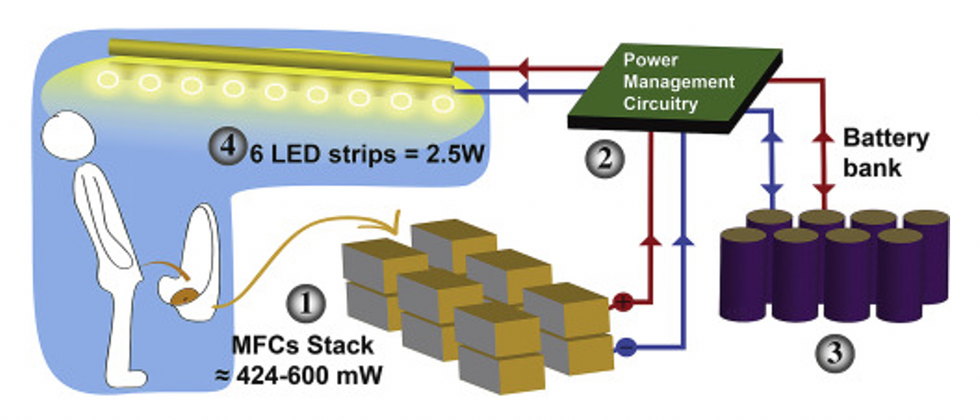
This image shows how the pee-powered system works. Pee feeds bacteria in the stack of fuel cells (1), which give off electrons (2) stored in parallel cylindrical cells (3). These cells are connected to a voltage regulator (4), which smooths out the electrical signal to ensure consistent power to the LED strips lighting the toilet.
Courtesy Ioannis Ieropoulos
Key to the long-term success of any urine reclamation effort, says Orner, is avoiding what he calls “parachute engineering”—when well-meaning scientists solve a problem with novel tech and then abandon it. “The way around that is to have either the need come from the community or to have an organization in a community that is committed to seeing a project operate and maintained,” he says.
Success with urine reclamation also depends on the economy. “If energy prices are low, it may not make sense to recover energy,” says Orner. “But right now, fertilizer prices worldwide are generally pretty high, so it may make sense to recover fertilizer and nutrients.” There are obstacles, too, such as few incentives for builders to incorporate urine recycling into new construction. And any hiccups like leaks or waste seepage will cost builders money and reputation. Right now, Orner says, the risks are just too high.
Despite the challenges, Ieropoulos envisions a future in which urine is passed through microbial fuel cells at wastewater treatment plants, retrofitted septic tanks, and building basements, and is then delivered to businesses to use as agricultural fertilizers. Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofitted into urban wastewater utilities where they can make electricity from the effluent. And unlike solar cells, which are a common target of theft in some areas, nobody wants to steal a bunch of pee.
When Ieropoulos’s team returned to wrap up their pilot project 18 months later, the school’s director begged them to leave the fuel cells in place—because they made a major difference in students’ lives. “We replaced it with a substantial photovoltaic panel,” says Ieropoulos, They couldn’t leave the units forever, he explained, because of intellectual property reasons—their funders worried about theft of both the technology and the idea. But the photovoltaic replacement could be stolen, too, leaving the girls in the dark.
The story repeated itself at another school, in Nairobi, Kenya, as well as in an informal settlement in Durban, South Africa. Each time, Ieropoulos vowed to return. Though the pandemic has delayed his promise, he is resolute about continuing his work—it is a moral and legal obligation. “We've made a commitment to ourselves and to the pupils,” he says. “That's why we need to go back.”
Urine as fertilizer
Modern day industrial systems perpetuate the broken cycle of nutrients. When plants grow, they use up nutrients the soil. We eat the plans and excrete some of the nutrients we pass them into rivers and oceans. As a result, farmers must keep fertilizing the fields while our waste keeps fertilizing the waterways, where the algae, overfertilized with nitrogen, phosphorous and other nutrients grows out of control, sucking up oxygen that other marine species need to live. Few global communities remain untouched by the related challenges this broken chain create: insufficient clean water, food, and energy, and too much human and animal waste.
The Rich Earth Institute in Vermont runs a community-wide urine nutrient recovery program, which collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms.
One solution to this broken cycle is reclaiming urine and returning it back to the land. The Rich Earth Institute in Vermont is one of several organizations around the world working to divert and save urine for agricultural use. “The urine produced by an adult in one day contains enough fertilizer to grow all the wheat in one loaf of bread,” states their website.
Notably, while urine is not entirely sterile, it tends to harbor fewer pathogens than feces. That’s largely because urine has less organic matter and therefore less food for pathogens to feed on, but also because the urinary tract and the bladder have built-in antimicrobial defenses that kill many germs. In fact, the Rich Earth Institute says it’s safe to put your own urine onto crops grown for home consumption. Nonetheless, you’ll want to dilute it first because pee usually has too much nitrogen and can cause “fertilizer burn” if applied straight without dilution. Other projects to turn urine into fertilizer are in progress in Niger, South Africa, Kenya, Ethiopia, Sweden, Switzerland, The Netherlands, Australia, and France.
Eleven years ago, the Institute started a program that collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms. By 2021, the program included 180 donors producing over 12,000 gallons of urine each year. This urine is helping to fertilize hay fields at four partnering farms. Orner, the West Virginia professor, sees it as a success story. “They've shown how you can do this right--implementing it at a community level scale."

