The Shiny–and Potentially Dangerous—New Tool for Predicting Human Behavior

Studies of twins have played an important role in determining that genetic differences play a role in the development of differences in behavior.
[Editor's Note: This essay is in response to our current Big Question, which we posed to experts with different perspectives: "How should DNA tests for intelligence be used, if at all, by parents and educators?"]
Imagine a world in which pregnant women could go to the doctor and obtain a simple inexpensive genetic test of their unborn child that would allow them to predict how tall he or she would eventually be. The test might also tell them the child's risk for high blood pressure or heart disease.
Can we use DNA not to understand, but to predict who is going to be intelligent or extraverted or mentally ill?
Even more remarkable -- and more dangerous -- the test might predict how intelligent the child would be, or how far he or she could be expected to go in school. Or heading further out, it might predict whether he or she will be an alcoholic or a teetotaler, or straight or gay, or… you get the idea. Is this really possible? If it is, would it be a good idea? Answering these questions requires some background in a scientific field called behavior genetics.
Differences in human behavior -- intelligence, personality, mental illness, pretty much everything -- are related to genetic differences among people. Scientists have known this for 150 years, ever since Darwin's half-cousin Francis Galton first applied Shakespeare's phrase, "Nature and Nurture" to the scientific investigation of human differences. We knew about the heritability of behavior before Mendel's laws of genetics had been re-discovered at the end of the last century, and long before the structure of DNA was discovered in the 1950s. How could discoveries about genetics be made before a science of genetics even existed?
The answer is that scientists developed clever research designs that allowed them to make inferences about genetics in the absence of biological knowledge about DNA. The best-known is the twin study: identical twins are essentially clones, sharing 100 percent of their DNA, while fraternal twins are essentially siblings, sharing half. To the extent that identical twins are more similar for some trait than fraternal twins, one can infer that heredity is playing a role. Adoption studies are even more straightforward. Is the personality of an adopted child more like the biological parents she has never seen, or the adoptive parents who raised her?
Twin and adoption studies played an important role in establishing beyond any reasonable doubt that genetic differences play a role in the development of differences in behavior, but they told us very little about how the genetics of behavior actually worked. When the human genome was finally sequenced in the early 2000s, and it became easier and cheaper to obtain actual DNA from large samples of people, scientists anticipated that we would soon find the genes for intelligence, mental illness, and all the other behaviors that were known to be "heritable" in a general way.
But to everyone's amazement, the genes weren't there. It turned out that there are thousands of genes related to any given behavior, so many that they can't be counted, and each one of them has such a tiny effect that it can't be tied to meaningful biological processes. The whole scientific enterprise of understanding the genetics of behavior seemed ready to collapse, until it was rescued -- sort of -- by a new method called polygenic scores, PGS for short. Polygenic scores abandon the old task of finding the genes for complex human behavior, replacing it with black-box prediction: can we use DNA not to understand, but to predict who is going to be intelligent or extraverted or mentally ill?
Prediction from observing parents works better, and is far easier and cheaper, than anything we can do with DNA.
PGS are the shiny new toy of human genetics. From a technological standpoint they are truly amazing, and they are useful for some scientific applications that don't involve making decisions about individual people. We can obtain DNA from thousands of people, estimate the tiny relationships between individual bits of DNA and any outcome we want — height or weight or cardiac disease or IQ — and then add all those tiny effects together into a single bell-shaped score that can predict the outcome of interest. In theory, we could do this from the moment of conception.
Polygenic scores for height already work pretty well. Physicians are debating whether the PGS for heart disease are robust enough to be used in the clinic. For some behavioral traits-- the most data exist for educational attainment -- they work well enough to be scientifically interesting, if not practically useful. For traits like personality or sexual orientation, the prediction is statistically significant but nowhere close to practically meaningful. No one knows how much better any of these predictions are likely to get.
Without a doubt, PGS are an amazing feat of genomic technology, but the task they accomplish is something scientists have been able to do for a long time, and in fact it is something that our grandparents could have done pretty well. PGS are basically a new way to predict a trait in an individual by using the same trait in the individual's parents — a way of observing that the acorn doesn't fall far from the tree.
The children of tall people tend to be tall. Children of excellent athletes are athletic; children of smart people are smart; children of people with heart disease are at risk, themselves. Not every time, of course, but that is how imperfect prediction works: children of tall parents vary in their height like anyone else, but on average they are taller than the rest of us. Prediction from observing parents works better, and is far easier and cheaper, than anything we can do with DNA.
But wait a minute. Prediction from parents isn't strictly genetic. Smart parents not only pass on their genes to their kids, but they also raise them. Smart families are privileged in thousands of ways — they make more money and can send their kids to better schools. The same is true for PGS.
The ability of a genetic score to predict educational attainment depends not only on examining the relationship between certain genes and how far people go in school, but also on every personal and social characteristic that helps or hinders education: wealth, status, discrimination, you name it. The bottom line is that for any kind of prediction of human behavior, separation of genetic from environmental prediction is very difficult; ultimately it isn't possible.
Still, experts are already discussing how to use PGS to make predictions for children, and even for embryos.
This is a reminder that we really have no idea why either parents or PGS predict as well or as poorly as they do. It is easy to imagine that a PGS for educational attainment works because it is summarizing genes that code for efficient neurological development, bigger brains, and swifter problem solving, but we really don't know that. PGS could work because they are associated with being rich, or being motivated, or having light skin. It's the same for predicting from parents. We just don't know.
Still, experts are already discussing how to use PGS to make predictions for children, and even for embryos.
For example, maybe couples could fertilize multiple embryos in vitro, test their DNA, and select the one with the "best" PGS on some trait. This would be a bad idea for a lot of reasons. Such scores aren't effective enough to be very useful to parents, and to the extent they are effective, it is very difficult to know what other traits might be selected for when parents try to prioritize intelligence or attractiveness. People will no doubt try it anyway, and as a matter of reproductive freedom I can't think of any way to stop them. Fortunately, the practice probably won't have any great impact one way or another.
That brings us to the ethics of PGS, particularly in the schools. Imagine that when a child enrolls in a public school, an IQ test is given to her biological parents. Children with low-IQ parents are statistically more likely to have low IQs themselves, so they could be assigned to less demanding classrooms or vocational programs. Hopefully we agree that this would be unethical, but let's think through why.
First of all, it would be unethical because we don't know why the parents have low IQs, or why their IQs predict their children's. The parents could be from a marginalized ethnic group, recognizable by their skin color and passed on genetically to their children, so discriminating based on a parent's IQ would just be a proxy for discriminating based on skin color. Such a system would be no more than a social scientific gloss on an old-fashioned program for perpetuating economic and cognitive privilege via the educational system.
People deserve to be judged on the basis of their own behavior, not a genetic test.
Assigning children to classrooms based on genetic testing would be no different, although it would have the slight ethical advantage of being less effective. The PGS for educational attainment could reflect brain-efficiency, but it could also depend on skin color, or economic advantage, or personality, or literally anything that is related in any way to economic success. Privileging kids with higher genetic scores would be no different than privileging children with smart parents. If schools really believe that a psychological trait like IQ is important for school placement, the sensible thing is to administer the children an actual IQ test – not a genetic test.
IQ testing has its own issues, of course, but at least it involves making decisions about individuals based on their own observable characteristics, rather than on characteristics of their parents or their genome. If decisions must be made, if resources must be apportioned, people deserve to be judged on the basis of their own behavior, the content of their character. Since it can't be denied that people differ in all sorts of relevant ways, this is what it means for all people to be created equal.
[Editor's Note: Read another perspective in the series here.]
Researchers are looking to engineer chocolate with less oil, which could reduce some of its detriments to health.
Creamy milk with velvety texture. Dark with sprinkles of sea salt. Crunchy hazelnut-studded chunks. Chocolate is a treat that appeals to billions of people worldwide, no matter the age. And it’s not only the taste, but the feel of a chocolate morsel slowly melting in our mouths—the smoothness and slipperiness—that’s part of the overwhelming satisfaction. Why is it so enjoyable?
That’s what an interdisciplinary research team of chocolate lovers from the University of Leeds School of Food Science and Nutrition and School of Mechanical Engineering in the U.K. resolved to study in 2021. They wanted to know, “What is making chocolate that desirable?” says Siavash Soltanahmadi, one of the lead authors of a new study about chocolates hedonistic quality.
Besides addressing the researchers’ general curiosity, their answers might help chocolate manufacturers make the delicacy even more enjoyable and potentially healthier. After all, chocolate is a billion-dollar industry. Revenue from chocolate sales, whether milk or dark, is forecasted to grow 13 percent by 2027 in the U.K. In the U.S., chocolate and candy sales increased by 11 percent from 2020 to 2021, on track to reach $44.9 billion by 2026. Figuring out how chocolate affects the human palate could up the ante even more.
Building a 3D tongue
The team began by building a 3D tongue to analyze the physical process by which chocolate breaks down inside the mouth.
As part of the effort, reported earlier this year in the scientific journal ACS Applied Materials and Interfaces, the team studied a large variety of human tongues with the intention to build an “average” 3D model, says Soltanahmadi, a lubrication scientist. When it comes to edible substances, lubrication science looks at how food feels in the mouth and can help design foods that taste better and have more satisfying texture or health benefits.
There are variations in how people enjoy chocolate; some chew it while others “lick it” inside their mouths.
Tongue impressions from human participants studied using optical imaging helped the team build a tongue with key characteristics. “Our tongue is not a smooth muscle, it’s got some texture, it has got some roughness,” Soltanahmadi says. From those images, the team came up with a digital design of an average tongue and, using 3D printed molds, built a “mimic tongue.” They also added elastomers—such as silicone or polyurethane—to mimic the roughness, the texture and the mechanical properties of a real tongue. “Wettability" was another key component of the 3D tongue, Soltanahmadi says, referring to whether a surface mixes with water (hydrophilic) or, in the case of oil, resists it (hydrophobic).
Notably, the resulting artificial 3D-tongues looked nothing like the human version, but they were good mimics. The scientists also created “testing kits” that produced data on various physical parameters. One such parameter was viscosity, the measure of how gooey a food or liquid is — honey is more viscous compared to water, for example. Another was tribology, which defines how slippery something is — high fat yogurt is more slippery than low fat yogurt; milk can be more slippery than water. The researchers then mixed chocolate with artificial saliva and spread it on the 3D tongue to measure the tribology and the viscosity. From there they were able to study what happens inside the mouth when we eat chocolate.
The team focused on the stages of lubrication and the location of the fat in the chocolate, a process that has rarely been researched.

The artificial 3D-tongues look nothing like human tongues, but they function well enough to do the job.
Courtesy Anwesha Sarkar and University of Leeds
The oral processing of chocolate
We process food in our mouths in several stages, Soltanahmadi says. And there is variation in these stages depending on the type of food. So, the oral processing of a piece of meat would be different from, say, the processing of jelly or popcorn.
There are variations with chocolate, in particular; some people chew it while others use their tongues to explore it (within their mouths), Soltanahmadi explains. “Usually, from a consumer perspective, what we find is that if you have a luxury kind of a chocolate, then people tend to start with licking the chocolate rather than chewing it.” The researchers used a luxury brand of dark chocolate and focused on the process of licking rather than chewing.
As solid cocoa particles and fat are released, the emulsion envelops the tongue and coats the palette creating a smooth feeling of chocolate all over the mouth. That tactile sensation is part of the chocolate’s hedonistic appeal we crave.
Understanding the make-up of the chocolate was also an important step in the study. “Chocolate is a composite material. So, it has cocoa butter, which is oil, it has some particles in it, which is cocoa solid, and it has sugars," Soltanahmadi says. "Dark chocolate has less oil, for example, and less sugar in it, most of the time."
The researchers determined that the oral processing of chocolate begins as soon as it enters a person’s mouth; it starts melting upon exposure to one’s body temperature, even before the tongue starts moving, Soltanahmadi says. Then, lubrication begins. “[Saliva] mixes with the oily chocolate and it makes an emulsion." An emulsion is a fluid with a watery (or aqueous) phase and an oily phase. As chocolate breaks down in the mouth, that solid piece turns into a smooth emulsion with a fatty film. “The oil from the chocolate becomes droplets in a continuous aqueous phase,” says Soltanahmadi. In other words, as solid cocoa particles and fat are released, the emulsion envelops the tongue and coats the palette, creating a smooth feeling of chocolate all over the mouth. That tactile sensation is part of the chocolate’s hedonistic appeal we crave, says Soltanahmadi.
Finding the sweet spot
After determining how chocolate is orally processed, the research team wanted to find the exact sweet spot of the breakdown of solid cocoa particles and fat as they are released into the mouth. They determined that the epicurean pleasure comes only from the chocolate's outer layer of fat; the secondary fatty layers inside the chocolate don’t add to the sensation. It was this final discovery that helped the team determine that it might be possible to produce healthier chocolate that would contain less oil, says Soltanahmadi. And therefore, less fat.
Rongjia Tao, a physicist at Temple University in Philadelphia, thinks the Leeds study and the concept behind it is “very interesting.” Tao, himself, did a study in 2016 and found he could reduce fat in milk chocolate by 20 percent. He believes that the Leeds researchers’ discovery about the first layer of fat being more important for taste than the other layer can inform future chocolate manufacturing. “As a scientist I consider this significant and an important starting point,” he says.
Chocolate is rich in polyphenols, naturally occurring compounds also found in fruits and vegetables, such as grapes, apples and berries. Research found that plant polyphenols can protect against cancer, diabetes and osteoporosis as well as cardiovascular ad neurodegenerative diseases.
Not everyone thinks it’s a good idea, such as chef Michael Antonorsi, founder and owner of Chuao Chocolatier, one of the leading chocolate makers in the U.S. First, he says, “cacao fat is definitely a good fat.” Second, he’s not thrilled that science is trying to interfere with nature. “Every time we've tried to intervene and change nature, we get things out of balance,” says Antonorsi. “There’s a reason cacao is botanically known as food of the gods. The botanical name is the Theobroma cacao: Theobroma in ancient Greek, Theo is God and Brahma is food. So it's a food of the gods,” Antonorsi explains. He’s doubtful that a chocolate made only with a top layer of fat will produce the same epicurean satisfaction. “You're not going to achieve the same sensation because that surface fat is going to dissipate and there is no fat from behind coming to take over,” he says.
Without layers of fat, Antonorsi fears the deeply satisfying experiential part of savoring chocolate will be lost. The University of Leeds team, however, thinks that it may be possible to make chocolate healthier - when consumed in limited amounts - without sacrificing its taste. They believe the concept of less fatty but no less slick chocolate will resonate with at least some chocolate-makers and consumers, too.
Chocolate already contains some healthful compounds. Its cocoa particles have “loads of health benefits,” says Soltanahmadi. Dark chocolate usually has more cocoa than milk chocolate. Some experts recommend that dark chocolate should contain at least 70 percent cocoa in order for it to offer some health benefit. Research has shown that the cocoa in chocolate is rich in polyphenols, naturally occurring compounds also found in fruits and vegetables, such as grapes, apples and berries. Research has shown that consuming plant polyphenols can be protective against cancer, diabetes and osteoporosis as well as cardiovascular and neurodegenerative diseases.
“So keeping the healthy part of it and reducing the oily part of it, which is not healthy, but is giving you that indulgence of it … that was the final aim,” Soltanahmadi says. He adds that the team has been approached by individuals in the chocolate industry about their research. “Everyone wants to have a healthy chocolate, which at the same time tastes brilliant and gives you that self-indulging experience.”
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
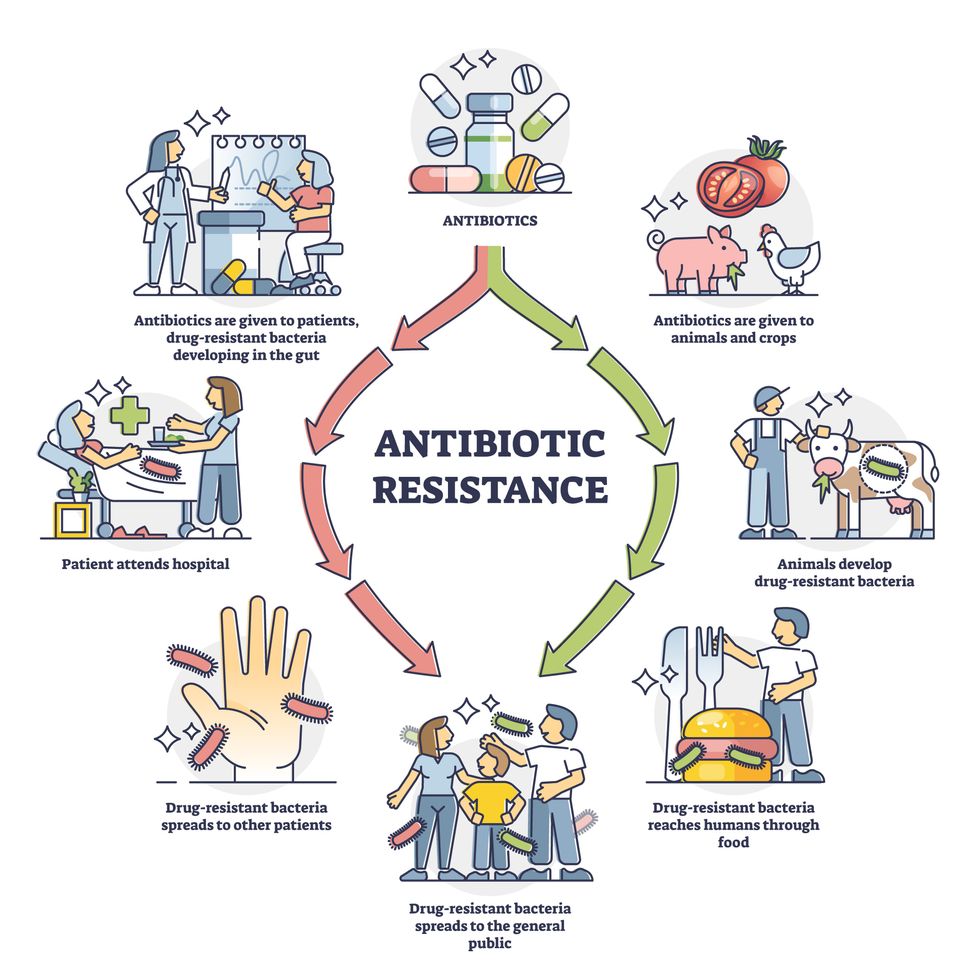
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”