The Troubling Reason I Obsessively Researched My Pregnancy

A pregnant woman in her third trimester.
At the end of my second trimester of pregnancy, I answered a call from an unknown number.
To be pregnant is to exist on a never-ending receiving line of advice, whether we want it or not.
"I know your due date is approaching," said a stranger at the other end of the line, completely freaking me out. She identified herself as being from Natera, a company that my doctor had used for genetic testing I had consented to months ago.
"Excuse me?" I said.
"Have you considered cord-blood banking?" she said.
"No, I'm not doing that," I said. I had read enough about cord-blood banking, the process of saving stem cell-containing blood from your baby's umbilical cord, to understand that my family was in the vast majority of those that would with extremely high likelihood derive no medical benefit from it. Of course, in the societally sanctioned spending spree that accompanies new parenthood, plenty of companies are happy to charge anyone hundreds if not thousands of dollars plus annual storage fees to collect and manage your cord blood.
"Why not? Have you considered all the bene—"
"I'm not doing it and I don't want to explain my decision," I said before hanging up. I would later learn I neglected to check a miniscule box on my testing consent forms at the doctor to opt out of solicitations. Still, I was angry that I was being telemarketed unnecessary and costly medical services by someone who had been trained to immediately call my judgment into question. I was annoyed that my doctor's office would allow such intrusions at all. When I asked my OB about it at my next visit, she told me there's no way Natera would have gotten my information from them. Apparently even she didn't realize what was on those forms.
The incident with Natera did nothing to heighten my trust of the medical establishment during my pregnancy. I was hardly alone. Almost every mom I knew had expressed a similar sentiment.
"I don't trust doctors," read the text of a loved one when I told her I would probably get an epidural after my doctor recommended getting one because, she said, it can help relax the pelvic muscles during labor. But this friend, a highly educated woman who had had done her research and had two unmedicated births, believed firmly otherwise. "Look it up," she said. Thus commenced more of the furious Googling I found myself doing multiple times a day since deciding I wanted to become pregnant.
To be pregnant is to exist on a never-ending receiving line of advice, whether we want it or not. Information presents to us from Google's never-out-of-reach search bar, friends and family eager to use our pregnancies as an excuse to recall their own, and the doctor's office, where the wisdom of medical professionals neatly comingles with brochures and free samples from myriad companies that would really, really like our business as new moms. Separating the "good" advice from the rest is a Herculean task that many pregnant women manage only with vigorous fact-finding missions of their own.
The medical community in America is poorly equipped to help women navigate the enormous pressures that come with birth and transitioning to motherhood.
Doing my research during pregnancy felt like a defense against the scary unknowns, overabundance of opinions, and disturbing marketing schemes that come with entering parenthood. The medical community in America is poorly equipped to help women navigate the enormous emotional and societal pressures that come with birth and transitioning to motherhood. Too much of what pregnant women experience at the doctor has to do with dated ideas about our care, mandated by tradition or a fear of being sued rather than medical necessity. These practices, like weigh-ins at every appointment or medically unnecessary C-sections (which are estimated to account, horrifically, for almost 50 percent of all C-sections performed in the U.S.), only heighten anxiety.
Meanwhile, things that might alleviate stress – like having thorough discussions about the kinds of interventions we might be asked to accept at the hospital during labor and delivery – are left to outside educators and doulas that insurance plans typically don't cover. The net effect isn't better health outcomes for mom and baby, but rather a normalized sense of distrust many American women feel toward their OBGYNs, and the burden of going to every appointment and the delivery room on the defensive. Instead of being wed to dated medical practices and tangled in America's new motherhood industrial complex, shouldn't our doctors, of all people, be our biggest advocates?
As soon as I found out I was pregnant, I devoured Expecting Better, by Emily Oster, an economist who embarked on her own fact-finding mission during her first pregnancy, predicated on the belief that the advice OBGYNs have been giving pregnant women for decades is out of date and unnecessarily restrictive. The book includes controversial stances, like that having small amounts of alcohol while pregnant is OK. (More recent research has called this view into question.) Oster writes that for the vast majority of pregnant women, it's perfectly fine to lie on your back, do sit-ups, and eat Brie — all things I was relieved to learn I wouldn't have to give up for nine months, despite the traditional advice, which my doctor also gave to me.
Oster recommends hiring a doula, based both on research and personal experience. It's a worthwhile investment for those who can afford it: according to one study, 20.4 percent of laboring women with doulas had C-sections compared with 34.2 percent of women without them. A doula can do many things for a pregnant client, including helping her write a birth plan, massaging her back in labor, and cheering her on, which is especially useful for women who plan to labor without pain medication. Use of doulas is on the rise; according to DONA International, the world's largest and oldest doula association, the number of doulas who have been certified to date is over 12,000, up from 2,000 in 2002.
But the most significant role a doula plays is that of patient advocate in the hospital. This is a profound commentary on the way the medical establishment handles childbirth, a medical event that 86 percent of women aged 40 to 44 had gone through as of 2016. Recognizing the maternal mortality crisis in the U.S., where women are far more likely to die as a result of childbirth than anywhere else in the developed world and black women are three times more likely to die in childbirth than white women, a few states now allow Medicaid to cover doulas. Can you imagine feeling the need to hire an independent non-medical care provider to help you run interference with your doctors and nurses for something like an appendectomy?
I wouldn't have been aware of all the imminent interventions during my labor if my doula hadn't told me about them. Things happen fast in the hospital and doctors and nurses may rush patients to consent before proceeding with things like breaking their water or hooking them up to an IV of Pitocin. Only because my husband and I had spent six hours in birth class — a suggestion by my doula — did I realize that I was empowered to say "no" to such procedures.
Expecting more trustworthy advice to come from my doctor than books or Google or even a doula hardly seems unreasonable.
Of course, we all feel immense pressure to become good parents, and questioning conventional medical wisdom is a natural response to that pressure. "Looking around at the world and saying, who am I as a parent? What is important to me? Who are the wise people? What do I think wisdom is? What is a good decision? If you're a certain type of introspective person, if you're really asking those questions, that's going to include like taking a second look at things that doctors, for example, say," says Koyuki Smith, a doula and birth educator.
Expecting more trustworthy advice to come from my doctor than books or Google or even a doula hardly seems unreasonable. Yet my doctor's office seemed more concerned with checking off a list of boxes rather than providing me with personalized care that might have relieved my understandable anxiety about my first birth. When I still hadn't gone into labor around the time of my due date, my doctor encouraged me to be induced because my baby appeared to be large. I declined but scheduled an induction to "hold my spot" around the 42-week mark.
When I asked what medication would be used for an induction if I had one and she said Cytotec, I told her I had read that drug could cause serious complications, but she dismissed my concerns after I told her they stemmed from a book I read on natural childbirth. The FDA's page on Cytotec isn't exactly reassuring.
The nurse who took me in triage after I went into labor a week past my due date practically scolded me for waiting to go into labor naturally instead of opting for induction sooner. My doula told her while I was struggling to speak through labor pains to get off my case about it. I hadn't even become a mom and I was already doing so many things "wrong." Because I had done my own reading, I felt confident that my choices weren't harming my baby or me.
Becoming a mom would be less daunting if the medical community found a way to help women navigate the pressures of motherhood instead of adding to them. "Our culture at large doesn't support women enough in the complicated emotions that are a part of this process," said Alexandra Saks, a reproductive psychologist and author of What No One Tells You: A Guide to Your Emotions From Pregnancy to Motherhood. "I hope that every practitioner that works with women around reproductive health prioritizes her emotions around her experience."
For many of us, that will mean doctors who help us understand the pros and cons of conventional advice, don't use their offices as marketing channels, and don't pressure women into medically unnecessary inductions. Moms should also receive more attention after delivery both in the hospital and after they get home; a single, quick postpartum visit at six weeks is not an adequate way to care for women recovering from the trauma of childbirth, nor is it an adequate way to ensure women are emotionally supported during the transition. While several people interrogated me about my mental health at the hospital and my doctor's office just before and after birth, if I had been concerned about postpartum depression, I can't imagine feeling comfortable enough in those moments to tell strangers filling out obligatory worksheets.
It also means figuring out how to talk to patients who are prone to Googling their pregnancies with gusto every single day. It would be impossible for many women to shun independent research during pregnancy altogether. But it would also be nice if our doctors didn't add to our impulse to do it.
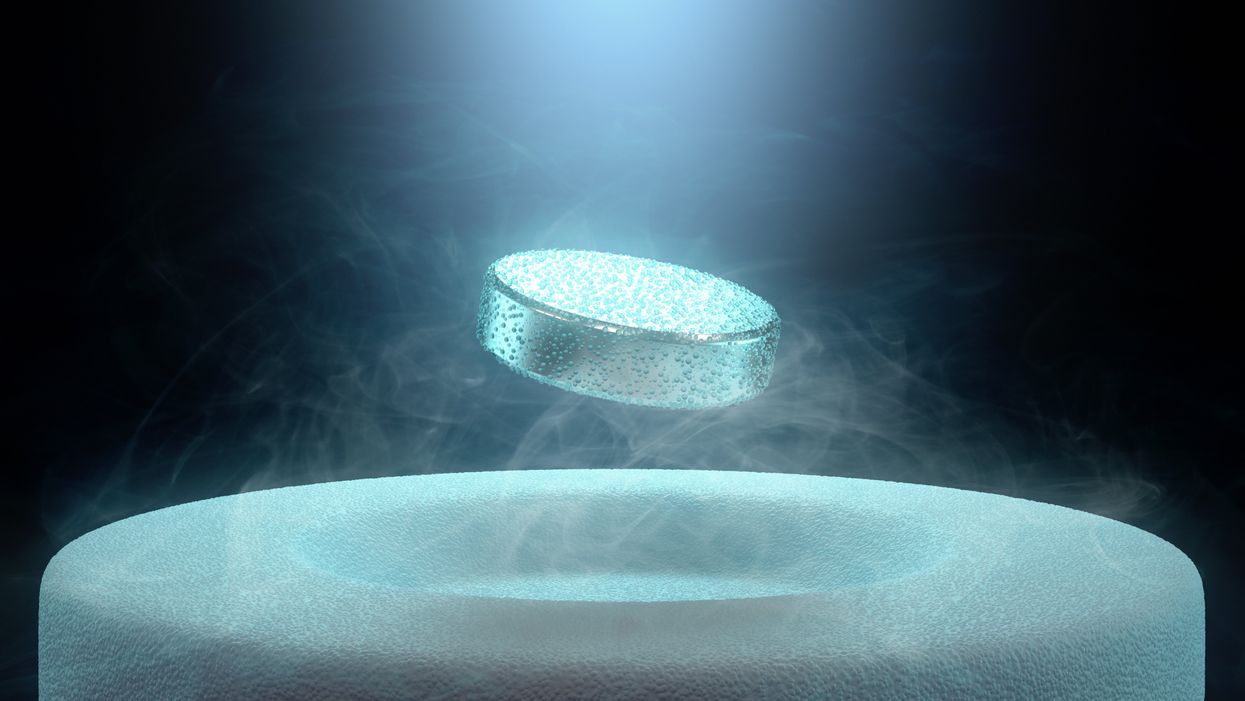
Researchers claimed they built a breakthrough superconductor. Social media shot it down almost instantly.
In July, South Korean scientists posted a paper finding they had achieved superconductivity - a claim that was debunked within days.
Harsh Mathur was a graduate physics student at Yale University in late 1989 when faculty announced they had failed to replicate claims made by scientists at the University of Utah and the University of Wolverhampton in England.
Such work is routine. Replicating or attempting to replicate the contraptions, calculations and conclusions crafted by colleagues is foundational to the scientific method. But in this instance, Yale’s findings were reported globally.
“I had a ringside view, and it was crazy,” recalls Mathur, now a professor of physics at Case Western Reserve University in Ohio.
Yale’s findings drew so much attention because initial experiments by Stanley Pons of Utah and Martin Fleischmann of Wolverhampton led to a startling claim: They were able to fuse atoms at room temperature – a scientific El Dorado known as “cold fusion.”
Nuclear fusion powers the stars in the universe. However, star cores must be at least 23.4 million degrees Fahrenheit and under extraordinary pressure to achieve fusion. Pons and Fleischmann claimed they had created an almost limitless source of power achievable at any temperature.
Like fusion, superconductivity can only be achieved in mostly impractical circumstances.
But about six months after they made their startling announcement, the pair’s findings were discredited by researchers at Yale and the California Institute of Technology. It was one of the first instances of a major scientific debunking covered by mass media.
Some scholars say the media attention for cold fusion stemmed partly from a dazzling announcement made three years prior in 1986: Scientists had created the first “superconductor” – material that could transmit electrical current with little or no resistance. It drew global headlines – and whetted the public’s appetite for announcements of scientific breakthroughs that could cause economic transformations.
But like fusion, superconductivity can only be achieved in mostly impractical circumstances: It must operate either at temperatures of at least negative 100 degrees Fahrenheit, or under pressures of around 150,000 pounds per square inch. Superconductivity that functions in closer to a normal environment would cut energy costs dramatically while also opening infinite possibilities for computing, space travel and other applications.
In July, a group of South Korean scientists posted material claiming they had created an iron crystalline substance called LK-99 that could achieve superconductivity at slightly above room temperature and at ambient pressure. The group partners with the Quantum Energy Research Centre, a privately-held enterprise in Seoul, and their claims drew global headlines.
Their work was also debunked. But in the age of internet and social media, the process was compressed from half-a-year into days. And it did not require researchers at world-class universities.
One of the most compelling critiques came from Derrick VanGennep. Although he works in finance, he holds a Ph.D. in physics and held a postdoctoral position at Harvard. The South Korean researchers had posted a video of a nugget of LK-99 in what they claimed was the throes of the Meissner effect – an expulsion of the substance’s magnetic field that would cause it to levitate above a magnet. Unless Hollywood magic is involved, only superconducting material can hover in this manner.
That claim made VanGennep skeptical, particularly since LK-99’s levitation appeared unenthusiastic at best. In fact, a corner of the material still adhered to the magnet near its center. He thought the video demonstrated ferromagnetism – two magnets repulsing one another. He mixed powdered graphite with super glue, stuck iron filings to its surface and mimicked the behavior of LK-99 in his own video, which was posted alongside the researchers’ video.
VanGennep believes the boldness of the South Korean claim was what led to him and others in the scientific community questioning it so quickly.
“The swift replication attempts stemmed from the combination of the extreme claim, the fact that the synthesis for this material is very straightforward and fast, and the amount of attention that this story was getting on social media,” he says.
But practicing scientists were suspicious of the data as well. Michael Norman, director of the Argonne Quantum Institute at the Argonne National Laboratory just outside of Chicago, had doubts immediately.
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication.
“It wasn’t a very polished paper,” Norman says of the Korean scientists’ work. That opinion was reinforced, he adds, when it turned out the paper had been posted online by one of the researchers prior to seeking publication in a peer-reviewed journal. Although Norman and Mathur say that is routine with scientific research these days, Norman notes it was posted by one of the junior researchers over the doubts of two more senior scientists on the project.
Norman also raises doubts about the data reported. Among other issues, he observes that the samples created by the South Korean researchers contained traces of copper sulfide that could inadvertently amplify findings of conductivity.
The lack of the Meissner effect also caught Mathur’s attention. “Ferromagnets tend to be unstable when they levitate,” he says, adding that the video “just made me feel unconvinced. And it made me feel like they hadn't made a very good case for themselves.”
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication. Despite being debunked, cold fusion claimants Pons and Fleischmann didn’t disappear. They moved their research to automaker Toyota’s IMRA laboratory in France, which along with the Japanese government spent tens of millions of dollars on their work before finally pulling the plug in 1998.
Fusion has since been created in laboratories, but being unable to reproduce the density of a star’s core would require excruciatingly high temperatures to achieve – about 160 million degrees Fahrenheit. A recently released Government Accountability Office report concludes practical fusion likely remains at least decades away.
However, like Pons and Fleischman, the South Korean researchers are not going anywhere. They claim that LK-99’s Meissner effect is being obscured by the fact the substance is both ferromagnetic and diamagnetic. They have filed for a patent in their country. But for now, those claims remain chimerical.
In the meantime, the consensus as to when a room temperature superconductor will be achieved is mixed. VenGennep – who studied the issue during his graduate and postgraduate work – puts the chance of creating such a superconductor by 2050 at perhaps 50-50. Mathur believes it could happen sooner, but adds that research on the topic has been going on for nearly a century, and that it has seen many plateaus.
“There's always this possibility that there's going to be something out there that we're going to discover unexpectedly,” Norman notes. The only certainty in this age of social media is that it will be put through the rigors of replication instantly.
Scientists implant brain cells to counter Parkinson's disease
In a recent research trial, patients with Parkinson's disease reported that their symptoms had improved after stem cells were implanted into their brains. Martin Taylor, far right, was diagnosed at age 32.
Martin Taylor was only 32 when he was diagnosed with Parkinson's, a disease that causes tremors, stiff muscles and slow physical movement - symptoms that steadily get worse as time goes on.
“It's horrible having Parkinson's,” says Taylor, a data analyst, now 41. “It limits my ability to be the dad and husband that I want to be in many cruel and debilitating ways.”
Today, more than 10 million people worldwide live with Parkinson's. Most are diagnosed when they're considerably older than Taylor, after age 60. Although recent research has called into question certain aspects of the disease’s origins, Parkinson’s eventually kills the nerve cells in the brain that produce dopamine, a signaling chemical that carries messages around the body to control movement. Many patients have lost 60 to 80 percent of these cells by the time they are diagnosed.
For years, there's been little improvement in the standard treatment. Patients are typically given the drug levodopa, a chemical that's absorbed by the brain’s nerve cells, or neurons, and converted into dopamine. This drug addresses the symptoms but has no impact on the course of the disease as patients continue to lose dopamine producing neurons. Eventually, the treatment stops working effectively.
BlueRock Therapeutics, a cell therapy company based in Massachusetts, is taking a different approach by focusing on the use of stem cells, which can divide into and generate new specialized cells. The company makes the dopamine-producing cells that patients have lost and inserts these cells into patients' brains. “We have a disease with a high unmet need,” says Ahmed Enayetallah, the senior vice president and head of development at BlueRock. “We know [which] cells…are lost to the disease, and we can make them. So it really came together to use stem cells in Parkinson's.”
In a phase 1 research trial announced late last month, patients reported that their symptoms had improved after a year of treatment. Brain scans also showed an increased number of neurons generating dopamine in patients’ brains.
Increases in dopamine signals
The recent phase 1 trial focused on deploying BlueRock’s cell therapy, called bemdaneprocel, to treat 12 patients suffering from Parkinson’s. The team developed the new nerve cells and implanted them into specific locations on each side of the patient's brain through two small holes in the skull made by a neurosurgeon. “We implant cells into the places in the brain where we think they have the potential to reform the neural networks that are lost to Parkinson's disease,” Enayetallah says. The goal is to restore motor function to patients over the long-term.
Five patients were given a relatively low dose of cells while seven got higher doses. Specialized brain scans showed evidence that the transplanted cells had survived, increasing the overall number of dopamine producing cells. The team compared the baseline number of these cells before surgery to the levels one year later. “The scans tell us there is evidence of increased dopamine signals in the part of the brain affected by Parkinson's,” Enayetallah says. “Normally you’d expect the signal to go down in untreated Parkinson’s patients.”
"I think it has a real chance to reverse motor symptoms, essentially replacing a missing part," says Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh.
The team also asked patients to use a specific type of home diary to log the times when symptoms were well controlled and when they prevented normal activity. After a year of treatment, patients taking the higher dose reported symptoms were under control for an average of 2.16 hours per day above their baselines. At the smaller dose, these improvements were significantly lower, 0.72 hours per day. The higher-dose patients reported a corresponding decrease in the amount of time when symptoms were uncontrolled, by an average of 1.91 hours, compared to 0.75 hours for the lower dose. The trial was safe, and patients tolerated the year of immunosuppression needed to make sure their bodies could handle the foreign cells.
Claire Bale, the associate director of research at Parkinson's U.K., sees the promise of BlueRock's approach, while noting the need for more research on a possible placebo effect. The trial participants knew they were getting the active treatment, and placebo effects are known to be a potential factor in Parkinson’s research. Even so, “The results indicate that this therapy produces improvements in symptoms for Parkinson's, which is very encouraging,” Bale says.
Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh, also finds the results intriguing. “I think it's excellent,” he says. “I think it has a real chance to reverse motor symptoms, essentially replacing a missing part.” However, it could take time for this therapy to become widely available, Kunath says, and patients in the late stages of the disease may not benefit as much. “Data from cell transplantation with fetal tissue in the 1980s and 90s show that cells did not survive well and release dopamine in these [late-stage] patients.”
Searching for the right approach
There's a long history of using cell therapy as a treatment for Parkinson's. About four decades ago, scientists at the University of Lund in Sweden developed a method in which they transferred parts of fetal brain tissue to patients with Parkinson's so that their nerve cells would produce dopamine. Many benefited, and some were able to stop their medication. However, the use of fetal tissue was highly controversial at that time, and the tissues were difficult to obtain. Later trials in the U.S. showed that people benefited only if a significant amount of the tissue was used, and several patients experienced side effects. Eventually, the work lost momentum.
“Like many in the community, I'm aware of the long history of cell therapy,” says Taylor, the patient living with Parkinson's. “They've long had that cure over the horizon.”
In 2000, Lorenz Studer led a team at the Memorial Sloan Kettering Centre, in New York, to find the chemical signals needed to get stem cells to differentiate into cells that release dopamine. Back then, the team managed to make cells that produced some dopamine, but they led to only limited improvements in animals. About a decade later, in 2011, Studer and his team found the specific signals needed to guide embryonic cells to become the right kind of dopamine producing cells. Their experiments in mice, rats and monkeys showed that their implanted cells had a significant impact, restoring lost movement.
Studer then co-founded BlueRock Therapeutics in 2016. Forming the most effective stem cells has been one of the biggest challenges, says Enayetallah, the BlueRock VP. “It's taken a lot of effort and investment to manufacture and make the cells at the right scale under the right conditions.” The team is now using cells that were first isolated in 1998 at the University of Wisconsin, a major advantage because they’re available in a virtually unlimited supply.
Other efforts underway
In the past several years, University of Lund researchers have begun to collaborate with the University of Cambridge on a project to use embryonic stem cells, similar to BlueRock’s approach. They began clinical trials this year.
A company in Japan called Sumitomo is using a different strategy; instead of stem cells from embryos, they’re reprogramming adults' blood or skin cells into induced pluripotent stem cells - meaning they can turn into any cell type - and then directing them into dopamine producing neurons. Although Sumitomo started clinical trials earlier than BlueRock, they haven’t yet revealed any results.
“It's a rapidly evolving field,” says Emma Lane, a pharmacologist at the University of Cardiff who researches clinical interventions for Parkinson’s. “But BlueRock’s trial is the first full phase 1 trial to report such positive findings with stem cell based therapies.” The company’s upcoming phase 2 research will be critical to show how effectively the therapy can improve disease symptoms, she added.
The cure over the horizon
BlueRock will continue to look at data from patients in the phase 1 trial to monitor the treatment’s effects over a two-year period. Meanwhile, the team is planning the phase 2 trial with more participants, including a placebo group.
For patients with Parkinson’s like Martin Taylor, the therapy offers some hope, though Taylor recognizes that more research is needed.

BlueRock Therapeutics
“Like many in the community, I'm aware of the long history of cell therapy,” he says. “They've long had that cure over the horizon.” His expectations are somewhat guarded, he says, but, “it's certainly positive to see…movement in the field again.”
"If we can demonstrate what we’re seeing today in a more robust study, that would be great,” Enayetallah says. “At the end of the day, we want to address that unmet need in a field that's been waiting for a long time.”
Editor's note: The company featured in this piece, BlueRock Therapeutics, is a portfolio company of Leaps by Bayer, which is a sponsor of Leaps.org. BlueRock was acquired by Bayer Pharmaceuticals in 2019. Leaps by Bayer and other sponsors have never exerted influence over Leaps.org content or contributors.