"There's a Bacteria For That"
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
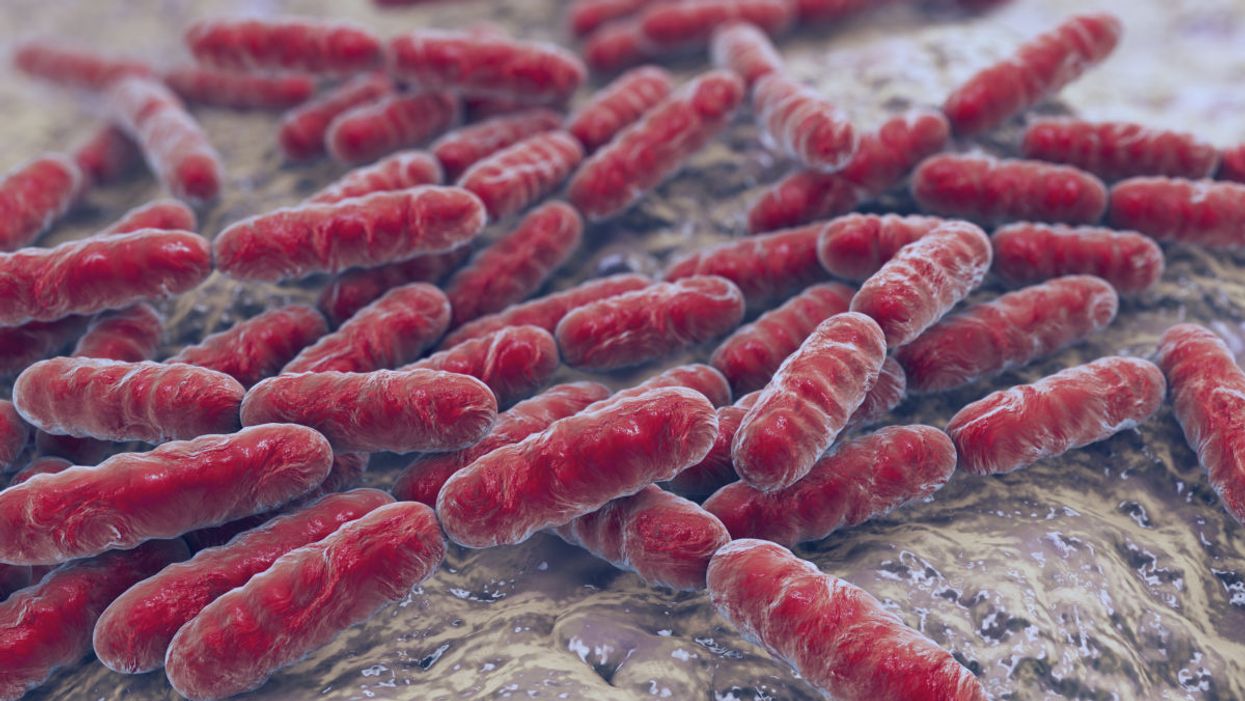
Bacteria Lactobacillus, gram-positive rod-shaped lactic acid bacteria which are part of normal flora of human intestine are used as probiotics and in yogurt production, close-up view. (Image copyright: Fotolia)
"There's an app for that." Get ready for a cutting-edge twist on this common phrase. In the life sciences, researchers in the field of synthetic biology are engineering microbes to execute specific tasks, like diagnosing gut inflammation, purifying dirty water, and cleaning up oil spills. Here are five academic and commercial projects underway now that will make you want to add the term "designer bacteria" to your vocab.
1) Bacteria that can sense, diagnose and treat disorders of the gut.
Dr. Pamela Silver at Harvard Medical School has engineered non-pathenogenic strains of E. Coli bacteria, which she calls "living diagnostics and therapeutics," to accurately sense whether an animal has been exposed to antibiotics and whether inflammation is present in its intestines.
Imagine a "living FitBit" that could report on your gut health in real time.
So how does it work? "The bacteria have a genetic switch like a light switch," she explains, "and when they are exposed to an antibiotic or an inflammatory response, the light switch flips to on and the bacteria turn color." In a study that Silver and her colleagues published earlier this year, the bacteria in mouse guts turned blue when exposed to the chemical tetrathionate, which is produced during inflammation. Then, when the animal excreted waste, its feces were also blue. For safety reasons, the excreted bacteria can additionally be programmed to self-destruct so as not to contaminate the environment.
The implications for human health go way beyond a non-invasive alternative to colonoscopies. Imagine "a living FitBit," Silver says with a laugh – a probiotic your doctor could prescribe that could colonize your gut to report on your intestinal health and your diet—and even treat pathogens at the same time. Another potential application is to deploy this new tool in the skin as a living sensor. "Your skin has a defined population of bacteria and those could be engineered to sense a lot," she says, such as pathological changes and toxic environmental exposures.
But one big social question in this emerging research remains how open the public and regulators will be to genetically modified organisms as drugs. Silver says that acceptance will require "patient advocacy, education, and showing these actually work. We have shown in an animal that it can work. So far, in humans, it's unclear."
"Live biotherapeutic products" is a whole new category of drug.
2) Bacteria that can treat a rare metabolic disease.
The startup company Synlogic, based in Cambridge, Mass., has designed an experimental pill containing a strain of E. Coli bacteria that can soak up excess ammonia in a person's stomach, treating those who suffer from toxic elevated blood ammonia levels. This condition, called hyperammonemia, can occur in those with chronic liver disease or genetic urea cycle disorders. The pill is genetically engineered to convert ammonia into a beneficial amino acid instead.
Just a few weeks ago, the company announced positive data from its Phase 1 trial, in which the pill was tested on a group of 52 healthy volunteers for the first time. The study was randomized, double-blind and placebo-controlled, which means that neither the researchers nor the subjects knew who was getting the active pill vs. a sham one. This design is the gold standard in clinical research because it overcomes bias and produces objective results. So far, the pill appears to be safe and well-tolerated, and the company plans to continue the next phase of testing in 2018. Synlogic's treatment stands to be the first of this category of therapy—called "live biotherapeutic products"—that will be scrutinized by the FDA when the time comes for possible market approval.
3) Bacteria that can be sprayed on land to clean up an oil spill.
"This is science fiction, but it's become a lot less science fiction in the last couple of years," says Floyd E. Romesberg, a professor of chemistry whose lab at the Scripps Research Institute in California is on the forefront of synthetic biology.
"We have literally increased the biology that cells can write stories with."
His lab has added two new letters to the code of life. At the most fundamental level, all life on Earth, including human, animal, and bacteria, relies on the four "letters" or chemical building blocks of A, T, C, and G to store biological information inside a cell and then retrieve it in the form of proteins that perform essential tasks. For the first time in history, Romesberg and his team have now developed an unnatural base pair—an X and a Y—capable of storing increased information.
"We have literally increased the biology that cells can write stories with," he says. "With new letters, you can write new words, new sentences, and you can tell new stories, as opposed to taking the limited vocabulary you have and trying to rearrange it."
The implications of his research are immense; applications range from developing therapeutic proteins as drugs, to bestowing cells with new properties, such as oxidizing oil after a spill. He imagines a future scenario in which, for example, specially engineered bacteria are sprayed on a beach, eat the oil for three generations of their life—less than a day—and then die off, since they will be unable to replicate their own DNA. Afterwards, the beach is clean.
"What we are struggling with now is the first steps toward doing that – the cell relying on unnatural information to survive, rather than doing something new yet," he says, "but that's where we are headed."
4) Bacteria that can deliver cancer-killing drugs inside tumors.
Researcher Jeff Hasty at UCSD has engineered a strain of Salmonella bacteria to penetrate cancer tumors and deliver drugs that stop their growth. His approach is especially clever because it solves a major problem in cancer drug delivery: chemotherapy relies on blood vessels for transit, but blood vessels don't exist deep inside tumors. Using this fact to his advantage, Hasty and his team designed bacteria that can sneak drugs all the way into a tumor and then self-destruct, taking the tumor down in the process.
So far, the treatment in mice has been successful; their tumors stopped growing after they were given the bacteria, and along with the use of chemotherapy, their life expectancy increased by half.
Many questions remain in terms of applicability to tumors in human beings, but the notion of a bacterial therapy remains a promising clinical approach for treating cancer in the future.
Craft beer experts couldn't tell the difference between beer brewed with regular vs. recycled water.
5) Bacteria that can convert wastewater into drinkable water.
Boston-based company Cambrian Innovation has a patented product called the EcoVolt MINI that uses microbes to generate energy through contact with electrodes. The company has collaborated with breweries across the country, taking their waste water and converting it to clean water and clean energy. Through the company's bioelectrochemical system, microbes eat the contaminants in the wastewater, and as a byproduct they produce methane, which can be converted to heat and power; in some cases, the process generates enough energy to send some back to the brewery.
"The main goal of the system is to produce cleaner water; the energy is an added product," explains Claire Aviles, Cambrian's marketing and communications manager.
The wastewater treatment is so effective that the water can be made suitable for reuse. One brewery client, for example, recently experimented with using the recycled water to brew a beer at a festival in California. They used the same recipe for two beers—one with typical city water and one with recycled water from Cambrian's system—and offered a side-by-side taste test to consumers and craft beer experts alike.
"Most people couldn't tell which was which," Aviles says.
In fact, most of the tasters preferred the beer brewed with the recycled water.
Turns out bacteria aren't always dirty after all.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.
Theupcoming vaccine is changing the way we look at treating one of the country’s deadliest cancers.
Last week, researchers at the University of Oxford announced that they have received funding to create a brand new way of preventing ovarian cancer: A vaccine. The vaccine, known as OvarianVax, will teach the immune system to recognize and destroy mutated cells—one of the earliest indicators of ovarian cancer.
Understanding Ovarian Cancer
Despite advancements in medical research and treatment protocols over the last few decades, ovarian cancer still poses a significant threat to women’s health. In the United States alone, more than 12,0000 women die of ovarian cancer each year, and only about half of women diagnosed with ovarian cancer survive five or more years past diagnosis. Unlike cervical cancer, there is no routine screening for ovarian cancer, so it often goes undetected until it has reached advanced stages. Additionally, the primary symptoms of ovarian cancer—frequent urination, bloating, loss of appetite, and abdominal pain—can often be mistaken for other non-cancerous conditions, delaying treatment.
An American woman has roughly a one percent chance of developing ovarian cancer throughout her lifetime. However, these odds increase significantly if she has inherited mutations in the BRCA1 or BRCA2 genes. Women who carry these mutations face a 46% lifetime risk for ovarian and breast cancers.
An Unlikely Solution
To address this escalating health concern, the organization Cancer Research UK has invested £600,000 over the next three years in research aimed at creating a vaccine, which would destroy cancerous cells before they have a chance to develop any further.
Researchers at the University of Oxford are at the forefront of this initiative. With funding from Cancer Research UK, scientists will use tissue samples from the ovaries and fallopian tubes of patients currently battling ovarian cancer. Using these samples, University of Oxford scientists will create a vaccine to recognize certain proteins on the surface of ovarian cancer cells known as tumor-associated antigens. The vaccine will then train that person’s immune system to recognize the cancer markers and destroy them.
The next step
Once developed, the vaccine will first be tested in patients with the disease, to see if their ovarian tumors will shrink or disappear. Then, the vaccine will be tested in women with the BRCA1 or BRCA2 mutations as well as women in the general population without genetic mutations, to see whether the vaccine can prevent the cancer altogether.
While the vaccine still has “a long way to go,” according to Professor Ahmed Ahmed, Director of Oxford University’s ovarian cancer cell laboratory, he is “optimistic” about the results.
“We need better strategies to prevent ovarian cancer,” said Ahmed in a press release from the University of Oxford. “Currently, women with BRCA1/2 mutations are offered surgery which prevents cancer but robs them of the chance to have children afterward.
Teaching the immune system to recognize the very early signs of cancer is a tough challenge. But we now have highly sophisticated tools which give us real insights into how the immune system recognizes ovarian cancer. OvarianVax could offer the solution.”

