This “Absolutely Tireless” Researcher Made an Important Breakthrough for Cancer Patients

Theo Roth.
After months of looking at dead cells under a microscope, Theo Roth finally glimpsed what he had been hoping to see—flickers of green. His method was working.
"If we can go into the cell and add in new code and instructions, now we can give it whatever new functions we want."
When Roth joined the laboratory of Alex Marson at the University of California, San Francisco in June 2016, he set to work trying to figure out a new way to engineer human T cells, a type of white blood cell that's an important part of the immune system. If he succeeded, the resulting approach could make it easier and faster for scientists to develop and test cell and gene therapies, new treatments that involve genetically reprogramming the body's own cells.
For decades, researchers have been using engineered viruses to bestow human cells with new genetic characteristics. These so-called viral vectors "infect" human cells, transferring whatever new genetic material scientists put into them. The idea is that this new DNA could give T cells a boost to better fight diseases like cancer and HIV.
Several successful clinical trials have used virally-modified human T cells, and in fact, the U.S. Food and Drug Administration last year approved two such groundbreaking cancer gene therapies, Kymriah and Yescarta. But the process of genetically manipulating cells with viruses is expensive and time-consuming. In addition, viruses tend to randomly insert DNA with little predictability.
"What Theo wanted to do was to paste in big sequences of DNA at a targeted site without viruses," says Marson, an associate professor of microbiology and immunology. "That would have the benefit of being able to rewrite a specific site in the genome and do it flexibly and quickly without having to make a new virus for every site you want to manipulate."
Scientists have for a while been interested in non-viral engineering methods, but T cells are fragile and notoriously difficult to work with.
Previously, Marson's lab had collaborated with CRISPR pioneer Jennifer Doudna and her team at the University of California, Berkeley to use an electrical pulse together with CRISPR components to knock out certain genes. They also found some success with inserting very small pieces of DNA into a targeted site.
But Roth, a 27-year-old graduate student at UCSF pursuing MD and PhD degrees, was determined to figure out how to paste in much bigger sequences of genetic information. Marson says it was an "ambitious" goal. Scientists had tried before, but found that stuffing large chunks of DNA into T cells would quickly kill them.
"If we can go into the cell and add in new code and instructions, now we can give it whatever new functions we want," Roth says. "If you can add in new DNA sequences at the site that you want, then you have a much greater capacity to generate a cell that's going to be therapeutic or curative for a disease."
"He has already made his mark on the field."
So Roth began experimenting with hundreds of different variables a week, trying to find the right conditions to allow him to engineer T cells without the need for viruses. To know if the technique was working, Roth and his colleagues used a green fluorescent protein that would be expressed in cells that had successfully been modified.
"We went from having a lot of dead cells that didn't have any green to having maybe 1 percent of them being green," Roth says. "At that stage we got really excited."
After nearly a year of testing, he and collaborators found a combination of T cell ratios and DNA quantity mixed with CRISPR and zaps of electricity that seemed to work. These electrical pulses, called electroporation, deliver a jolt to cells that makes their membranes temporarily more permeable, allowing the CRISPR system to slip through. Once inside cells, CRISPR seeks out a specific place in the genome and makes a programmed, precise edit.
Roth and his colleagues used the approach to repair a genetic defect in T cells taken from children with a rare autoimmune disease and also to supercharge T cells so that they'd seek out and selectively kill human cancer cells while leaving healthy cells intact. In mice transplanted with human melanoma tissue, the edited T cells went to straight to the cancerous cells and attacked them. The findings were published in Nature in July.
Marson and Roth think even a relatively small number of modified T cells could be effective at treating some cancers, infections, and autoimmune diseases.
Roth is now working with the Parker Institute for Cancer Immunotherapy in San Francisco to engineer cells to treat a variety of cancers and hopefully commercialize his technique. Fred Ramsdell, vice president at the Parker Institute, says he's impressed by Roth's work. "He has already made his mark on the field."
Right now, there's a huge manufacturing backlog for viruses. If researchers want to start a clinical trial to test a new gene or cell therapy, they often have to wait a year to get the viruses they need.
"I think the biggest immediate impact is that it will lower the cost of a starting an early phase clinical trial."
Ramsdell says what Roth's findings allow researchers to do is engineer T cells quickly and more efficiently, cutting the time it takes to make them from several months to just a few weeks. That will allow researchers to develop and test several potential therapies in the lab at once.
"I think the biggest immediate impact is that it will lower the cost of a starting an early phase clinical trial," Roth says.
This isn't the first time Roth's work has been in the spotlight. As an undergraduate at Stanford University, he made significant contributions to traumatic brain injury research by developing a mouse model for observing the brain's cellular response to a concussion. He started the research, which was also published in Nature, the summer before entering college while he was an intern in Dorian McGavern's lab at the National Institutes of Health.
When Roth entered UCSF as a graduate student, his scientific interests shifted.
"It's definitely a big leap" from concussion research, says McGavern, who still keeps in touch with Roth. But he says he's not surprised about Roth's path. "He's absolutely tireless when it comes to the pursuit of science."
Roth says he's optimistic about the potential for gene and cell therapies to cure patients. "I want to try to figure out what one of the next therapies we should put into patients should be."
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
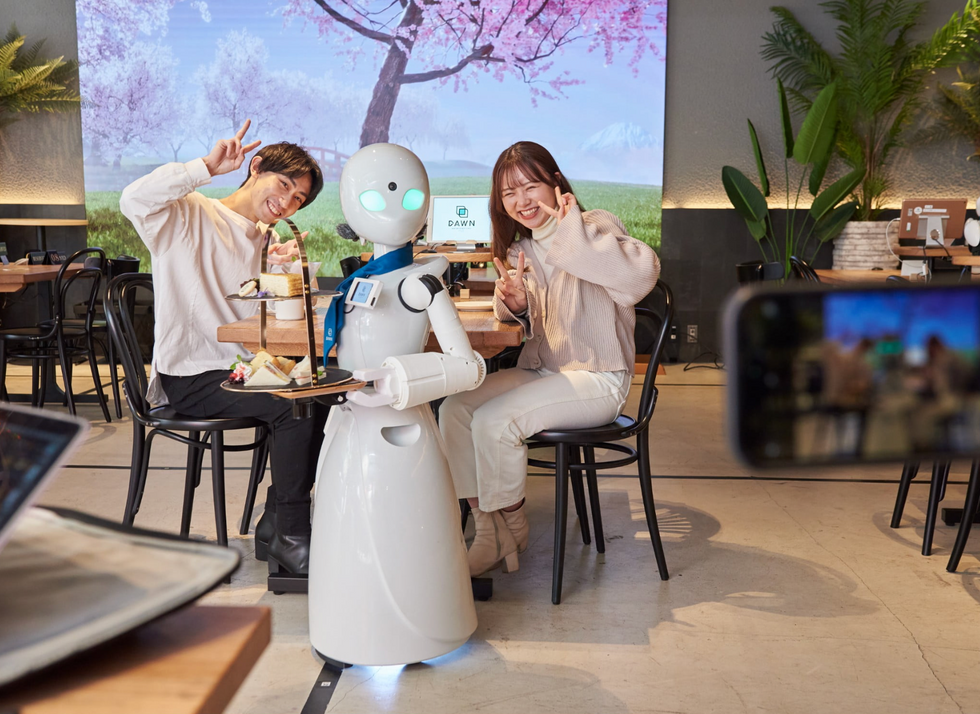
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

