Three Big Biotech Ideas to Watch in 2020—And Beyond

Body-on-a-chip, prime editing, and gut microbes all are poised to make a big impact in 2020.
1. Happening Now: Body-on-a-Chip Technology Is Enabling Safer Drug Trials and Better Cancer Research
Researchers have increasingly used the technology known as "lab-on-a-chip" or "organ-on-a-chip" to test the effects of pharmaceuticals, toxins, and chemicals on humans. Rather than testing on animals, which raises ethical concerns and can sometimes be inaccurate, and human-based clinical trials, which can be expensive and difficult to iterate, scientists turn to tiny, micro-engineered chips—about the size of a thumb drive.
It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip.
The chips are lined with living samples of human cells, which mimic the physiology and mechanical forces experienced by cells inside the human body, down to blood flow and breathing motions; the functions of organs ranging from kidneys and lungs to skin, eyes, and the blood-brain barrier.
A more recent—and potentially even more useful—development takes organ-on-a-chip technology to the next level by integrating several chips into a "body-on-a-chip." Since human organs don't work in isolation, seeing how they all react—and interact—once a foreign element has been introduced can be crucial to understanding how a certain treatment will or won't perform. Dr. Shyni Varghese, a MEDx investigator at the Duke University School of Medicine, is one of the researchers working with these systems in order to gain a more nuanced understanding of how multiple different organs react to the same stimuli.
Her lab is working on "tumor-on-a-chip" models, which can not only show the progression and treatment of cancer, but also model how other organs would react to immunotherapy and other drugs. "The effect of drugs on different organs can be tested to identify potential side effects," Varghese says. In addition, these models can help the researchers figure out how cancers grow and spread, as well as how to effectively encourage immune cells to move in and attack a tumor.
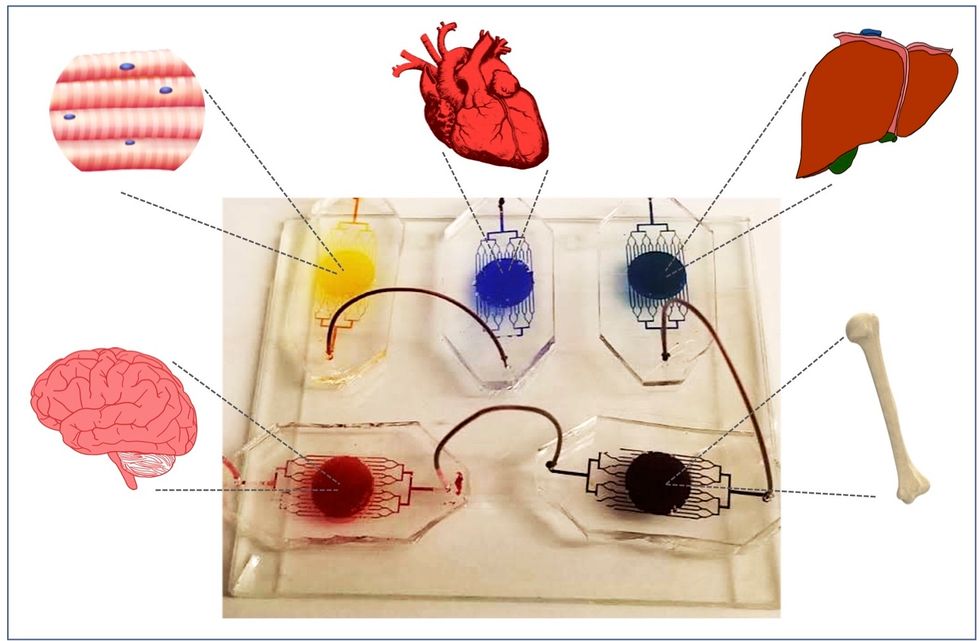
One body-on-a-chip used by Dr. Varghese's lab tracks the interactions of five organs—brain, heart, liver, muscle, and bone.
As their research progresses, Varghese and her team are looking for ways to maintain the long-term function of the engineered organs. In addition, she notes that this kind of research is not just useful for generalized testing; "organ-on-chip technologies allow patient-specific analyses, which can be used towards a fundamental understanding of disease progression," Varghese says. It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip for safety, efficacy, and potential side effects before writing a prescription.
2. Happening Soon: Prime Editing Will Have the Power to "Find and Replace" Disease-Causing Genes
Biochemist David Liu made industry-wide news last fall when he and his lab at MIT's Broad Institute, led by Andrew Anzalone, published a paper on prime editing: a new, more focused technology for editing genes. Prime editing is a descendant of the CRISPR-Cas9 system that researchers have been working with for years, and a cousin to Liu's previous innovation—base editing, which can make a limited number of changes to a single DNA letter at a time.
By contrast, prime editing has the potential to make much larger insertions and deletions; it also doesn't require the tweaked cells to divide in order to write the changes into the DNA, which could make it especially suitable for central nervous system diseases, like Parkinson's.
Crucially, the prime editing technique has a much higher efficiency rate than the older CRISPR system, and a much lower incidence of accidental insertions or deletions, which can make dangerous changes for a patient.
It also has a very broad potential range: according to Liu, 89% of the pathogenic mutations that have been collected in ClinVar (a public archive of human variations) could, in principle, be treated with prime editing—although he is careful to note that correcting a single genetic mutation may not be sufficient to fully treat a genetic disease.
Figuring out just how prime editing can be used most effectively and safely will be a long process, but it's already underway. The same day that Liu and his team posted their paper, they also made the basic prime editing constructs available for researchers around the world through Addgene, a plasmid repository, so that others in the scientific community can test out the technique for themselves. It might be years before human patients will see the results, and in the meantime, significant bioethical questions remain about the limits and sociological effects of such a powerful gene-editing tool. But in the long fight against genetic diseases, it's a huge step forward.
3. Happening When We Fund It: Focusing on Microbiome Health Could Help Us Tackle Social Inequality—And Vice Versa
The past decade has seen a growing awareness of the major role that the microbiome, the microbes present in our digestive tract, play in human health. Having a less-healthy microbiome is correlated with health risks like diabetes and depression, and interventions that target gut health, ranging from kombucha to fecal transplants, have cropped up with increasing frequency.
New research from the University of Maine's Dr. Suzanne Ishaq takes an even broader view, arguing that low-income and disadvantaged populations are less likely to have healthy, diverse gut bacteria, and that increasing access to beneficial microorganisms is an important juncture of social justice and public health.
"Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
"Typically, having a more diverse bacterial community is associated with health, and having fewer different species is associated with illness and may leave you open to infection from bacteria that are good at exploiting opportunities," Ishaq says.
Having a healthy biome doesn't mean meeting one fixed ratio of gut bacteria, since different combinations of microbes can generate roughly similar results when they work in concert. Generally, "good" microbes are the ones that break down fiber and create the byproducts that we use for energy, or ones like lactic acid bacteria that work to make microbials and keep other bacteria in check. The microbial universe in your gut is chaotic, Ishaq says. "Microbes in your gut interact with each other, with you, with your food, or maybe they don't interact at all and pass right through you." Overall, it's tricky to name specific microbial communities that will make or break someone's health.
There are important corollaries between environment and biome health, though, which Ishaq points out: Living in urban environments reduces microbial exposure, and losing the microorganisms that humans typically source from soil and plants can reduce our adaptive immunity and ability to fight off conditions like allergies and asthma. Access to green space within cities can counteract those effects, but in the U.S. that access varies along income, education, and racial lines. Likewise, lower-income communities are more likely to live in food deserts or areas where the cheapest, most convenient food options are monotonous and low in fiber, further reducing microbial diversity.
Ishaq also suggests other areas that would benefit from further study, like the correlation between paid family leave, breastfeeding, and gut microbiota. There are technical and ethical challenges to direct experimentation with human populations—but that's not what Ishaq sees as the main impediment to future research.
"The biggest roadblock is money, and the solution is also money," she says. "Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
That means investment in things we already understand to improve public health, like better education and healthcare, green space, and nutritious food. It also means funding ambitious, interdisciplinary research that will investigate the connections between urban infrastructure, housing policy, social equity, and the millions of microbes keeping us company day in and day out.
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

