To Make Science Engaging, We Need a Sesame Street for Adults

A new kind of television series could establish the baseline narratives for novel science like gene editing, quantum computing, or artificial intelligence.
This article is part of the magazine, "The Future of Science In America: The Election Issue," co-published by LeapsMag, the Aspen Institute Science & Society Program, and GOOD.
In the mid-1960s, a documentary producer in New York City wondered if the addictive jingles, clever visuals, slogans, and repetition of television ads—the ones that were captivating young children of the time—could be harnessed for good. Over the course of three months, she interviewed educators, psychologists, and artists, and the result was a bonanza of ideas.
Perhaps a new TV show could teach children letters and numbers in short animated sequences? Perhaps adults and children could read together with puppets providing comic relief and prompting interaction from the audience? And because it would be broadcast through a device already in almost every home, perhaps this show could reach across socioeconomic divides and close an early education gap?
Soon after Joan Ganz Cooney shared her landmark report, "The Potential Uses of Television in Preschool Education," in 1966, she was prototyping show ideas, attracting funding from The Carnegie Corporation, The Ford Foundation, and The Corporation for Public Broadcasting, and co-founding the Children's Television Workshop with psychologist Lloyd Morrisett. And then, on November 10, 1969, informal learning was transformed forever with the premiere of Sesame Street on public television.
For its first season, Sesame Street won three Emmy Awards and a Peabody Award. Its star, Big Bird, landed on the cover of Time Magazine, which called the show "TV's gift to children." Fifty years later, it's hard to imagine an approach to informal preschool learning that isn't Sesame Street.
And that approach can be boiled down to one word: Entertainment.
Despite decades of evidence from Sesame Street—one of the most studied television shows of all time—and more research from social science, psychology, and media communications, we haven't yet taken Ganz Cooney's concepts to heart in educating adults. Adults have news programs and documentaries and educational YouTube channels, but no Sesame Street. So why don't we? Here's how we can design a new kind of television to make science engaging and accessible for a public that is all too often intimidated by it.
We have to start from the realization that America is a nation of high-school graduates. By the end of high school, students have decided to abandon science because they think it's too difficult, and as a nation, we've made it acceptable for any one of us to say "I'm not good at science" and offload thinking to the ones who might be. So, is it surprising that a large number of Americans are likely to believe in conspiracy theories like the 25% that believe the release of COVID-19 was planned, the one in ten who believe the Moon landing was a hoax, or the 30–40% that think the condensation trails of planes are actually nefarious chemtrails? If we're meeting people where they are, the aim can't be to get the audience from an A to an A+, but from an F to a D, and without judgment of where they are starting from.
There's also a natural compulsion for a well-meaning educator to fill a literacy gap with a barrage of information, but this is what I call "factsplaining," and we know it doesn't work. And worse, it can backfire. In one study from 2014, parents were provided with factual information about vaccine safety, and it was the group that was already the most averse to vaccines that uniquely became even more averse.
Why? Our social identities and cognitive biases are stubborn gatekeepers when it comes to processing new information. We filter ideas through pre-existing beliefs—our values, our religions, our political ideologies. Incongruent ideas are rejected. Congruent ideas, no matter how absurd, are allowed through. We hear what we want to hear, and then our brains justify the input by creating narratives that preserve our identities. Even when we have all the facts, we can use them to support any worldview.
But social science has revealed many mechanisms for hijacking these processes through narrative storytelling, and this can form the foundation of a new kind of educational television.
Could new television series establish the baseline narratives for novel science like gene editing, quantum computing, or artificial intelligence?
As media creators, we can reject factsplaining and instead construct entertaining narratives that disrupt cognitive processes. Two-decade-old research tells us when people are immersed in entertaining fiction narratives, they loosen their defenses, opening a path for new information, editing attitudes, and inspiring new behavior. Where news about hot-button issues like climate change or vaccination might trigger resistance or a backfire effect, fiction can be crafted to be absorbing and, as a result, persuasive.
But the narratives can't be stuffed with information. They must be simplified. If this feels like the opposite of what an educator should be doing, it is possible to reduce the complexity of information, without oversimplification, through "exemplification," a framing device to tell the stories of individuals in specific circumstances that can speak to the greater issue without needing to explain it all. It's a technique you've seen used in biopics. The Discovery Channel true-crime miniseries Manhunt: Unabomber does many things well from a science storytelling perspective, including exemplifying the virtues of the scientific method through a character who argues for a new field of science, forensic linguistics, to catch one of the most notorious domestic terrorists in U.S. history.
We must also appeal to the audience's curiosity. We know curiosity is such a strong driver of human behavior that it can even counteract the biases put up by one's political ideology around subjects like climate change. If we treat science information like a product—and we should—advertising research tells us we can maximize curiosity though a Goldilocks effect. If the information is too complex, your show might as well be a PowerPoint presentation. If it's too simple, it's Sesame Street. There's a sweet spot for creating intrigue about new information when there's a moderate cognitive gap.
The science of "identification" tells us that the more the main character is endearing to a viewer, the more likely the viewer will adopt the character's worldview and journey of change. This insight further provides incentives to craft characters reflective of our audiences. If we accept our biases for what they are, we can understand why the messenger becomes more important than the message, because, without an appropriate messenger, the message becomes faint and ineffective. And research confirms that the stereotype-busting doctor-skeptic Dana Scully of The X-Files, a popular science-fiction series, was an inspiration for a generation of women who pursued science careers.
With these directions, we can start making a new kind of television. But is television itself still the right delivery medium? Americans do spend six hours per day—a quarter of their lives—watching video. And even with the rise of social media and apps, science-themed television shows remain popular, with four out of five adults reporting that they watch shows about science at least sometimes. CBS's The Big Bang Theory was the most-watched show on television in the 2017–2018 season, and Cartoon Network's Rick & Morty is the most popular comedy series among millennials. And medical and forensic dramas continue to be broadcast staples. So yes, it's as true today as it was in the 1980s when George Gerbner, the "cultivation theory" researcher who studied the long-term impacts of television images, wrote, "a single episode on primetime television can reach more people than all science and technology promotional efforts put together."
We know from cultivation theory that media images can shape our views of scientists. Quick, picture a scientist! Was it an old, white man with wild hair in a lab coat? If most Americans don't encounter research science firsthand, it's media that dictates how we perceive science and scientists. Characters like Sheldon Cooper and Rick Sanchez become the model. But we can correct that by representing professionals more accurately on-screen and writing characters more like Dana Scully.
Could new television series establish the baseline narratives for novel science like gene editing, quantum computing, or artificial intelligence? Or could new series counter the misinfodemics surrounding COVID-19 and vaccines through more compelling, corrective narratives? Social science has given us a blueprint suggesting they could. Binge-watching a show like the surreal NBC sitcom The Good Place doesn't replace a Ph.D. in philosophy, but its use of humor plants the seed of continued interest in a new subject. The goal of persuasive entertainment isn't to replace formal education, but it can inspire, shift attitudes, increase confidence in the knowledge of complex issues, and otherwise prime viewers for continued learning.
[Editor's Note: To read other articles in this special magazine issue, visit the beautifully designed e-reader version.]
A Doctor Who Treated His Own Rare Disease Is Tracking COVID-19 Treatments Hiding In Plain Sight
Dr. David Fajgenbaum looking through a microscope at his lab.
In late March, just as the COVID-19 pandemic was ramping up in the United States, David Fajgenbaum, a physician-scientist at the University of Pennsylvania, devised a 10-day challenge for his lab: they would sift through 1,000 recently published scientific papers documenting cases of the deadly virus from around the world, pluck out the names of any drugs used in an attempt to cure patients, and track the treatments and their outcomes in a database.
Before late 2019, no one had ever had to treat this exact disease before, which meant all treatments would be trial and error. Fajgenbaum, a pioneering researcher in the field of drug repurposing—which prioritizes finding novel uses for existing drugs, rather than arduously and expensively developing new ones for each new disease—knew that physicians around the world would be embarking on an experimental journey, the scale of which would be unprecedented. His intention was to briefly document the early days of this potentially illuminating free-for-all, as a sidebar to his primary field of research on a group of lymph node disorders called Castleman disease. But now, 11 months and 29,000 scientific papers later, he and his team of 22 are still going strong.
They're running a publicly accessible database called the CORONA Project (COvid19 Registry of Off-label & New Agents) that to date tracks 400 different COVID-19 treatments that have been tried somewhere in the world, along with the frequency of their use, and the outcomes.
"There's so many drugs being used all over the place, in different ways, with different outcomes," says Fajgenbaum. "We're trying to add some order to the madness."
20,000 people have accessed the registry—other physicians and researchers, those in the pharmaceutical industry, and even curious lay people—and the data are now being shared with the U.S. Food and Drug Administration in the hopes of launching large-scale trials that would lead to approving a constellation of treatment options for COVID-19 faster than any new drugs could come online.
"What David's group has done with the CORONA Project is on a scale that I don't think has ever been seen before," says Heather Stone, a health science policy analyst at the FDA who specializes in drug repurposing. She was not involved in establishing the project, but is now working with its data. "To collect and collate that information and make it openly accessible is a massive feat, and a huge benefit to the medical community," she says.
On a Personal Mission
In the science and medical world, Fajgenbaum lives a dual existence: he is both researcher and subject, physician and patient. In July 2010, when he was a healthy and physically fit 25-year-old finishing medical school, he began living through what would become a recurring, unprovoked, and overzealous immune response that repeatedly almost killed him.
His lymph nodes were inflamed; his liver, kidneys, and bone marrow were faltering; and he was dead tired all the time. At first his doctors mistook his mysterious illness for lymphoma, but his inflamed lymph nodes were merely a red herring. A month after his initial hospitalization, pathologists at Mayo Clinic finally diagnosed him with idiopathic multicentric Castleman disease—a particularly ruthless form of a class of lymph node disorders that doesn't just attack one part of the body, but many, and has no known cause. It's a rare diagnosis within an already rare set of disorders. Only about 1,500 Americans a year receive the same diagnosis.
Without many options for treatment, Fajgenbaum underwent recurring rounds of chemotherapy. Each time, the treatment would offer temporary respite from Castleman symptoms, but bring the full spate of chemotherapy side effects. And it wasn't a sustainable treatment for the long haul. Regularly dousing a person's cells in unmitigated toxicity was about as elegant a solution to Fajgenbaum's disease as bulldozing a house in response to a toaster fire. The fire might go out (though not necessarily), but the house would be destroyed.
A swirl of exasperation and doggedness finally propelled Fajgenbaum to take on a crucial question himself: Among all of the already FDA-approved drugs on the market, was there something out there, labeled for another use, that could beat back Castleman disease and that he could tolerate long-term? After months of research, he discovered the answer: sirolimus, a drug normally prescribed to patients receiving a kidney transplant, could be used to suppress his overactive immune system with few known side effects to boot.
Fajgenbaum became hellbent on devoting his practice and research to making similar breakthroughs for others. He founded the Castleman Disease Collaborative Network, to coordinate the research of others studying this bewildering disease, and directs a laboratory consumed with studying cytokine storms—out-of-control immune responses characterized by the body's release of cytokines, proteins that the immune system secretes and uses to communicate with and direct other cells.
In the spring of 2020, when cytokine storms emerged as a hallmark of the most severe and deadly cases of COVID-19, Fajgenbaum's ears perked up. Although SARS-CoV-2 itself was novel, Fajgenbaum already had almost a decade of experience battling the most severe biological forces it brought. Only this time, he thought, it might actually be easier to pinpoint a treatment—unlike Castleman disease, which has no known cause, at least here a virus was clearly the instigator.
"Because [a drug] looks promising, we need to do a well-designed, large randomized controlled trial to really investigate whether this drug works or not ... We don't use that to say, 'You should take it.'"
Thinking Beyond COVID
The week of March 13, when the World Health Organization declared COVID-19 a pandemic, Fajgenbaum found himself hoping that someone would make the same connection and apply the research to COVID. "Then like a minute later I was like, 'Why am I hoping that someone, somewhere, either follows our footsteps, or has a similar background to us? Maybe we just need to do it," he says. And the CORONA Project was born—first as a 10-day exercise, and later as the robust, interactive tool it now is.
All of the 400 treatments in the CORONA database are examples of repurposed drugs, or off-label uses: physicians are prescribing drugs to treat COVID that have been approved for a different disease. There are no bonafide COVID treatments, only inferences. The goal for people like Fajgenbaum and Stone is to identify potential treatments for further study and eventual official approval, so that physicians can treat the disease with a playbook in hand. When it works, drug repurposing opens up a way to move quickly: A range of treatments could be available to patients within just a few years of a totally new virus entering our reality compared with the 12 - 19 years new drug development takes.
"Companies for many decades have explored the use of their products for not just a single indication but often for many indications," says Stone. "'Supplemental approvals' are all essentially examples of drug repurposing, we just didn't call it that. The challenge, I think, is to explore those opportunities more comprehensively and systematically to really try to understand the full breadth of potential activity of any drug or molecule."
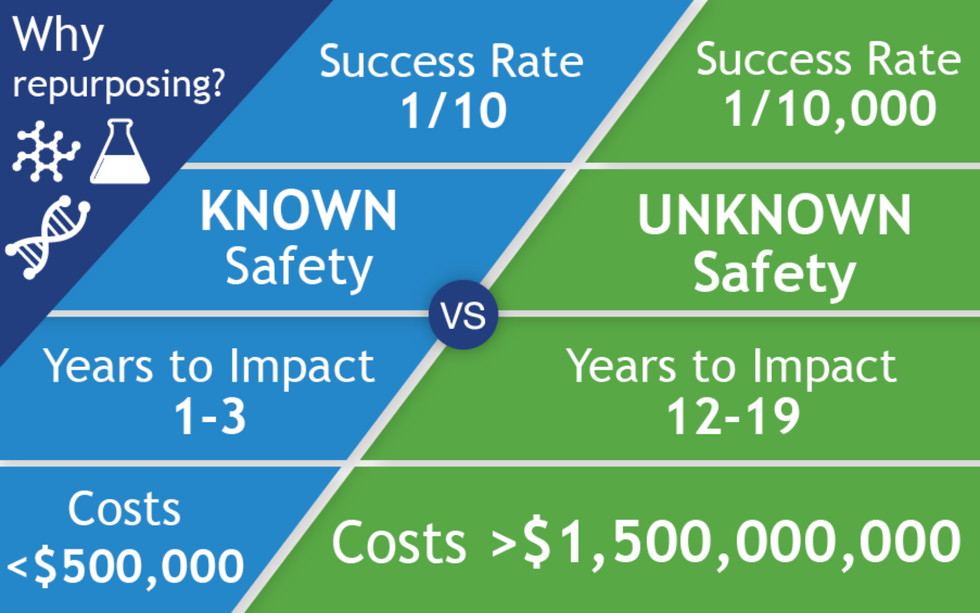
The left column shows the path of a repurposed drug, and on the right is the path of a newly discovered and developed drug.
Cures Within Reach
In Fajgenbaum's primary work, promising drugs stand out easily. For a disease like Castleman, where improvement almost never occurs on its own, any improvement that follows a treatment can pretty clearly be attributed to that treatment. But Fajgenbaum says tracking COVID outcomes is less straightforward since "the vast majority of people will get better, whether they take steroids or they take Skittles." That's why the intent of the database is to identify promising treatments only to generate hypotheses and fruitful clinical trials, not to offer full-throated treatment recommendations. Within the registry, Fajgenbaum considers a drug promising if it's being used in humans, not just in lab animals, and a significant proportion of cases report patient improvement.
"It's that sort of combination of rock-solid randomized controlled trial data, plus anecdotal retrospective data, that we combine to say, 'Wow, this drug looks more promising than another,'" says Fajgenbaum. "Because it looks promising, we need to do a well-designed, large randomized controlled trial to really investigate whether this drug works or not ... We don't use that to say, 'You should take it.'"
Experts say that the search for repurposed drugs to treat COVID could have implications for rare diseases in general. Rare diseases, of which Castleman is one, affect 400 million people around the world. 95% of them don't have a tailor-made, FDA-approved drug treatment. Developing one is a lengthy and often prohibitively expensive process. If only a dozen people will benefit from and buy a drug, it's not often worth it to pharmaceutical companies to spend millions of dollars making them. On occasion when they do, however, that overhead shows up in the price tag: the top 10 most expensive drugs in the world are all for rare diseases, often making them inaccessible to patients. Identifying new clinical uses for drugs that already exist is critical for opening a trap door out of a cycle that prioritizes profits over health outcomes.
"COVID is an interesting case where it's demonstrated that when the scientific and medical community really focuses all of its efforts and talents on a single problem, a solution can be identified and in a much faster time period than has ever historically been the case," says Stone. "I certainly wish it hadn't taken a pandemic to do that, but I think it does have lessons for the future in terms of our ability to accomplish things that we might have previously not thought were possible"—for example, mainstreaming the idea of drug repurposing as a treatment tool, even long after the pandemic subsides.
A Confounding Virus
The FDA declined to comment on what drugs it was fast-tracking for trials, but Fajgenbaum says that based on the CORONA Project's data, which includes data from smaller trials that have already taken place, he feels there are three drugs that seem the most clearly and broadly promising for large-scale studies. Among them are dexamethasone, which is a steroid with anti-inflammatory effects, and baricitinib, a rheumatoid arthritis drug, both of which have enabled the sickest COVID-19 patients to bounce back by suppressing their immune systems. The third most clearly promising drug is heparin, a blood thinner, which a recent trial showed to be most helpful when administered at a full dose, more so than at a small, preventative dose. (On the flipside, Fajgenbaum says "it's a little sad" that in the database you can see hydroxychloroquine is still the most-prescribed drug being tried as a COVID treatment around the world, despite over the summer being debunked widely as an effective treatment, and continuously since then.)
One of the confounding attributes of SARS-CoV-2 is its ability to cause such a huge spectrum of outcomes. It's unlikely a silver bullet treatment will emerge under that reality, so the database also helps surface drugs that seem most promising for a specific population. Fluvoxamine, a selective serotonin reuptake inhibitor used to treat obsessive compulsive disorder, showed promise in the recovery of outpatients—those who were sick, but not severely enough to be hospitalized. Tocilizumab, which was actually developed for Castleman disease, the disease Fajgenbaum is managing, was initially written off as a COVID treatment because it failed to benefit large portions of hospitalized patients, but now seems to be effective if used on intensive care unit patients within 24 hours of admission—these are some of the sickest patients with the highest risk of dying.
Other than fluvoxamine, most of the drugs labeled as promising do skew toward targeting hospitalized patients, more than outpatients. One reason, Fajgenbaum says, is that "if you're in a hospital it's very easy to give you a drug and to track you, and there are very objective measurements as to whether you die, you progress to a ventilator, etc." Tracking outpatients is far more difficult, especially when folks have been routinely asked to stay home, quarantine, and free up hospital resources if they're experiencing only mild symptoms.
But the other reason for the skew is because COVID is very unlike most other diseases in terms of the human immune response the virus triggers. For example, if oncology treatments show some benefit to people with the highest risk of dying, then they usually work extremely well if administered in the earlier stages of a cancer diagnosis. Across many diseases, this dialing backward is a standard approach to identifying promising treatments. With COVID, all of that reasoning has proven moot.
As we've seen over the last year, COVID cases often start as asymptomatic, and remain that way for days, indicating the body is mounting an incredibly weak immune response initially. Then, between days five and 14, as if trying to make up for lost time, the immune system overcompensates by launching a major inflammatory response, which in the sickest patient can lead to the type of cytokine storms that helped Fajgenbaum realize his years of Castleman research might be useful during this public health crisis. Because of this phased response, you can't apply the same treatment logic to all cases.
"In COVID, drugs that work late tend to not work if given early, and drugs that work early tend to not work if given late," says Fajgenbaum. "Generally this … is not a commonplace thing for a virus."
"There are drugs that are literally sitting in every single hospital pharmacy in the country that, if a study shows it's effective, can be deployed that evening to patients on a massive scale."
This see-sawing necessitates tracking a constellation of drugs that might work for different stages of the disease as a patient moves from the weak immune response stage into the overzealous immune response.
"COVID is difficult, compared to other diseases, because there are so many different levels of disease severity, and recovery at different rates," says Stone, the FDA researcher. "That makes it hard to see the patterns or signals and it makes it very important to collect very, very large numbers of cases in order to really reliably identify signals."
This particular moment in the pandemic feels like a massive tipping point, or the instant a tiny pinprick of light finally appeared at the end of the tunnel: several vaccines are already here, with more on the way imminently. In the U.S., more than 65 million doses of the vaccine have been administered, and positive COVID cases are finally falling back to levels not seen since October. On the hopeful surface, it might seem a strange moment to be preparing to launch trials that will validate treatments for a virus it seems the U.S. may finally be beating back. But at best, Americans are still months away from reaching herd immunity through vaccination, and new circulating variants may threaten to upend our fragile progress.
"In the meantime, there are drugs that are literally sitting in every single hospital pharmacy in the country that, if a study shows it's effective, can be deployed that evening to patients on a massive scale. It wouldn't have to be newly produced, it wouldn't have to be shipped, it's literally there already," says Fajgenbaum. "The idea that you can save a lot of lives by finding things that are just already there I think is really compelling, given how many people are going to die over these next few months."
Even after that, not everyone can or will be vaccinated, and, as the Wall Street Journal recently reported, "The pathogen will circulate for years, or even decades, leaving society to coexist with Covid-19 much as it does with other endemic diseases like flu, measles, and HIV." Neither vaccines, personal behavior, or treatments alone is a panacea against the virus, but together they might be.
"It's important to explore all avenues in this public health emergency, and drug repurposing can continue to play a role as the pandemic continues and evolves," says Stone. "I think COVID variants in particular are a big concern at the moment, and therefore continuing to investigate new therapeutics, even as the vaccines roll out, will continue to be a priority."
How a Nobel-Prize Winner Fought Her Family, Nazis, and Bombs to Change our Understanding of Cells Forever
Rita Levi-Montalcini survived the Nazis and eventually won a Nobel Prize for her work to understand why certain cells grow so quickly.
When Rita Levi-Montalcini decided to become a scientist, she was determined that nothing would stand in her way. And from the beginning, that determination was put to the test. Before Levi-Montalcini became a Nobel Prize-winning neurobiologist, the first to discover and isolate a crucial chemical called Neural Growth Factor (NGF), she would have to battle both the sexism within her own family as well as the racism and fascism that was slowly engulfing her country
Levi-Montalcini was born to two loving parents in Turin, Italy at the turn of the 20th century. She and her twin sister, Paola, were the youngest of the family's four children, and Levi-Montalcini described her childhood as "filled with love and reciprocal devotion." But while her parents were loving, supportive and "highly cultured," her father refused to let his three daughters engage in any schooling beyond the basics. "He loved us and had a great respect for women," she later explained, "but he believed that a professional career would interfere with the duties of a wife and mother."
At age 20, Levi-Montalcini had finally had enough. "I realized that I could not possibly adjust to a feminine role as conceived by my father," she is quoted as saying, and asked his permission to finish high school and pursue a career in medicine. When her father reluctantly agreed, Levi-Montalcini was ecstatic: In just under a year, she managed to catch up on her mathematics, graduate high school, and enroll in medical school in Turin.
By 1936, Levi-Montalcini had graduated medical school at the top of her class and decided to stay on at the University of Turin as a research assistant for histologist and human anatomy professor Guiseppe Levi. Levi-Montalcini started studying nerve cells and nerve fibers – the tiny, slender tendrils that are threaded throughout our nerves and that determine what information each nerve can transmit. But it wasn't long before another enormous obstacle to her scientific career reared its head.
Science Under a Fascist Regime
Two years into her research assistant position, Levi-Montalcini was fired, along with every other "non-Aryan Italian" who held an academic or professional career, thanks to a series of antisemitic laws passed by Italy's then-leader Benito Mussolini. Forced out of her academic position, Levi-Montalcini went to Belgium for a fellowship at a neurological institute in Brussels – but then was forced back to Turin when the German army invaded.
Levi-Montalcini decided to keep researching. She and Guiseppe Levi built a makeshift lab in Levi-Montalcini's apartment, borrowing chicken eggs from local farmers and using sewing needles to dissect them. By dissecting the chicken embryos from her bedroom laboratory, she was able to see how nerve fibers formed and died. The two continued this research until they were interrupted again – this time, by British air raids. Levi-Montalcini fled to a country cottage to continue her research, and then two years later was forced into hiding when the German army invaded Italy. Levi-Montalcini and her family assumed different identities and lived with non-Jewish friends in Florence to survive the Holocaust. Despite all of this, Levi-Montalcini continued her work, dissecting chicken embryos from her hiding place until the end of the war.
"The discovery of NGF really changed the world in which we live, because now we knew that cells talk to other cells, and that they use soluble factors. It was hugely important."
A Post-War Discovery
Several years after the war, when Levi-Montalcini was once again working at the University of Turin, a German embryologist named Viktor Hamburger invited her to Washington University in St. Louis. Hamburger was impressed by Levi-Montalcini's research with her chicken embryos, and secured an opportunity for her to continue her work in America. The invitation would "change the course of my life," Levi-Montalcini would later recall.
During her fellowship, Montalcini grew tumors in mice and then transferred them to chick embryos in order to see how it would affect the chickens. To her surprise, she noticed that introducing the tumor samples would cause nerve fibers to grow rapidly. From this, Levi-Montalcini discovered and was able to isolate a protein that she determined was able to cause this rapid growth. She later named this Nerve Growth Factor, or NGF.
From there, Levi-Montalcini and her team launched new experiments to test NGF, injecting it and repressing it to see the effect it had in a test subject's body. When the team injected NGF into embryonic mice, they observed nerve growth, as well as the mouse pups developing faster – their eyes opening earlier and their teeth coming in sooner – than the untreated group. When the team purified the NGF extract, however, it had no effect, leading the team to believe that something else in the crude extract of NGF was influencing the growth of the newborn mice. Stanley Cohen, Levi-Montalcini's colleague, identified another growth factor called EGF – epidermal growth factor – that caused the mouse pups' eyes and teeth to grow so quickly.
Levi-Montalcini continued to experiment with NGF for the next several decades at Washington University, illuminating how NGF works in our body. When Levi-Montalcini injected newborn mice with an antiserum for NGF, for example, her team found that it "almost completely deprived the animals of a sympathetic nervous system." Other experiments done by Levi-Montalcini and her colleagues helped show the role that NGF plays in other important biological processes, such as the regulation of our immune system and ovulation.
"The discovery of NGF really changed the world in which we live, because now we knew that cells talk to other cells, and that they use soluble factors. It was hugely important," said Bill Mobley, Chair of the Department of Neurosciences at the University of California, San Diego School of Medicine.
Her Lasting Legacy
After years of setbacks, Levi-Montalcini's groundbreaking work was recognized in 1986, when she was awarded the Nobel Prize in Medicine for her discovery of NGF (Cohen, her colleague who discovered EGF, shared the prize). Researchers continue to study NGF even to this day, and the continued research has been able to increase our understanding of diseases like HIV and Alzheimer's.
Levi-Montalcini never stopped researching either: In January 2012, at the age of 102, Levi-Montalcini published her last research paper in the journal PNAS, making her the oldest member of the National Academy of Science to do so. Before she died in December 2012, she encouraged other scientists who would suffer setbacks in their careers to keep pursuing their passions. "Don't fear the difficult moments," Levi-Montalcini is quoted as saying. "The best comes from them."

