To Make Science Engaging, We Need a Sesame Street for Adults

A new kind of television series could establish the baseline narratives for novel science like gene editing, quantum computing, or artificial intelligence.
This article is part of the magazine, "The Future of Science In America: The Election Issue," co-published by LeapsMag, the Aspen Institute Science & Society Program, and GOOD.
In the mid-1960s, a documentary producer in New York City wondered if the addictive jingles, clever visuals, slogans, and repetition of television ads—the ones that were captivating young children of the time—could be harnessed for good. Over the course of three months, she interviewed educators, psychologists, and artists, and the result was a bonanza of ideas.
Perhaps a new TV show could teach children letters and numbers in short animated sequences? Perhaps adults and children could read together with puppets providing comic relief and prompting interaction from the audience? And because it would be broadcast through a device already in almost every home, perhaps this show could reach across socioeconomic divides and close an early education gap?
Soon after Joan Ganz Cooney shared her landmark report, "The Potential Uses of Television in Preschool Education," in 1966, she was prototyping show ideas, attracting funding from The Carnegie Corporation, The Ford Foundation, and The Corporation for Public Broadcasting, and co-founding the Children's Television Workshop with psychologist Lloyd Morrisett. And then, on November 10, 1969, informal learning was transformed forever with the premiere of Sesame Street on public television.
For its first season, Sesame Street won three Emmy Awards and a Peabody Award. Its star, Big Bird, landed on the cover of Time Magazine, which called the show "TV's gift to children." Fifty years later, it's hard to imagine an approach to informal preschool learning that isn't Sesame Street.
And that approach can be boiled down to one word: Entertainment.
Despite decades of evidence from Sesame Street—one of the most studied television shows of all time—and more research from social science, psychology, and media communications, we haven't yet taken Ganz Cooney's concepts to heart in educating adults. Adults have news programs and documentaries and educational YouTube channels, but no Sesame Street. So why don't we? Here's how we can design a new kind of television to make science engaging and accessible for a public that is all too often intimidated by it.
We have to start from the realization that America is a nation of high-school graduates. By the end of high school, students have decided to abandon science because they think it's too difficult, and as a nation, we've made it acceptable for any one of us to say "I'm not good at science" and offload thinking to the ones who might be. So, is it surprising that a large number of Americans are likely to believe in conspiracy theories like the 25% that believe the release of COVID-19 was planned, the one in ten who believe the Moon landing was a hoax, or the 30–40% that think the condensation trails of planes are actually nefarious chemtrails? If we're meeting people where they are, the aim can't be to get the audience from an A to an A+, but from an F to a D, and without judgment of where they are starting from.
There's also a natural compulsion for a well-meaning educator to fill a literacy gap with a barrage of information, but this is what I call "factsplaining," and we know it doesn't work. And worse, it can backfire. In one study from 2014, parents were provided with factual information about vaccine safety, and it was the group that was already the most averse to vaccines that uniquely became even more averse.
Why? Our social identities and cognitive biases are stubborn gatekeepers when it comes to processing new information. We filter ideas through pre-existing beliefs—our values, our religions, our political ideologies. Incongruent ideas are rejected. Congruent ideas, no matter how absurd, are allowed through. We hear what we want to hear, and then our brains justify the input by creating narratives that preserve our identities. Even when we have all the facts, we can use them to support any worldview.
But social science has revealed many mechanisms for hijacking these processes through narrative storytelling, and this can form the foundation of a new kind of educational television.
Could new television series establish the baseline narratives for novel science like gene editing, quantum computing, or artificial intelligence?
As media creators, we can reject factsplaining and instead construct entertaining narratives that disrupt cognitive processes. Two-decade-old research tells us when people are immersed in entertaining fiction narratives, they loosen their defenses, opening a path for new information, editing attitudes, and inspiring new behavior. Where news about hot-button issues like climate change or vaccination might trigger resistance or a backfire effect, fiction can be crafted to be absorbing and, as a result, persuasive.
But the narratives can't be stuffed with information. They must be simplified. If this feels like the opposite of what an educator should be doing, it is possible to reduce the complexity of information, without oversimplification, through "exemplification," a framing device to tell the stories of individuals in specific circumstances that can speak to the greater issue without needing to explain it all. It's a technique you've seen used in biopics. The Discovery Channel true-crime miniseries Manhunt: Unabomber does many things well from a science storytelling perspective, including exemplifying the virtues of the scientific method through a character who argues for a new field of science, forensic linguistics, to catch one of the most notorious domestic terrorists in U.S. history.
We must also appeal to the audience's curiosity. We know curiosity is such a strong driver of human behavior that it can even counteract the biases put up by one's political ideology around subjects like climate change. If we treat science information like a product—and we should—advertising research tells us we can maximize curiosity though a Goldilocks effect. If the information is too complex, your show might as well be a PowerPoint presentation. If it's too simple, it's Sesame Street. There's a sweet spot for creating intrigue about new information when there's a moderate cognitive gap.
The science of "identification" tells us that the more the main character is endearing to a viewer, the more likely the viewer will adopt the character's worldview and journey of change. This insight further provides incentives to craft characters reflective of our audiences. If we accept our biases for what they are, we can understand why the messenger becomes more important than the message, because, without an appropriate messenger, the message becomes faint and ineffective. And research confirms that the stereotype-busting doctor-skeptic Dana Scully of The X-Files, a popular science-fiction series, was an inspiration for a generation of women who pursued science careers.
With these directions, we can start making a new kind of television. But is television itself still the right delivery medium? Americans do spend six hours per day—a quarter of their lives—watching video. And even with the rise of social media and apps, science-themed television shows remain popular, with four out of five adults reporting that they watch shows about science at least sometimes. CBS's The Big Bang Theory was the most-watched show on television in the 2017–2018 season, and Cartoon Network's Rick & Morty is the most popular comedy series among millennials. And medical and forensic dramas continue to be broadcast staples. So yes, it's as true today as it was in the 1980s when George Gerbner, the "cultivation theory" researcher who studied the long-term impacts of television images, wrote, "a single episode on primetime television can reach more people than all science and technology promotional efforts put together."
We know from cultivation theory that media images can shape our views of scientists. Quick, picture a scientist! Was it an old, white man with wild hair in a lab coat? If most Americans don't encounter research science firsthand, it's media that dictates how we perceive science and scientists. Characters like Sheldon Cooper and Rick Sanchez become the model. But we can correct that by representing professionals more accurately on-screen and writing characters more like Dana Scully.
Could new television series establish the baseline narratives for novel science like gene editing, quantum computing, or artificial intelligence? Or could new series counter the misinfodemics surrounding COVID-19 and vaccines through more compelling, corrective narratives? Social science has given us a blueprint suggesting they could. Binge-watching a show like the surreal NBC sitcom The Good Place doesn't replace a Ph.D. in philosophy, but its use of humor plants the seed of continued interest in a new subject. The goal of persuasive entertainment isn't to replace formal education, but it can inspire, shift attitudes, increase confidence in the knowledge of complex issues, and otherwise prime viewers for continued learning.
[Editor's Note: To read other articles in this special magazine issue, visit the beautifully designed e-reader version.]
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
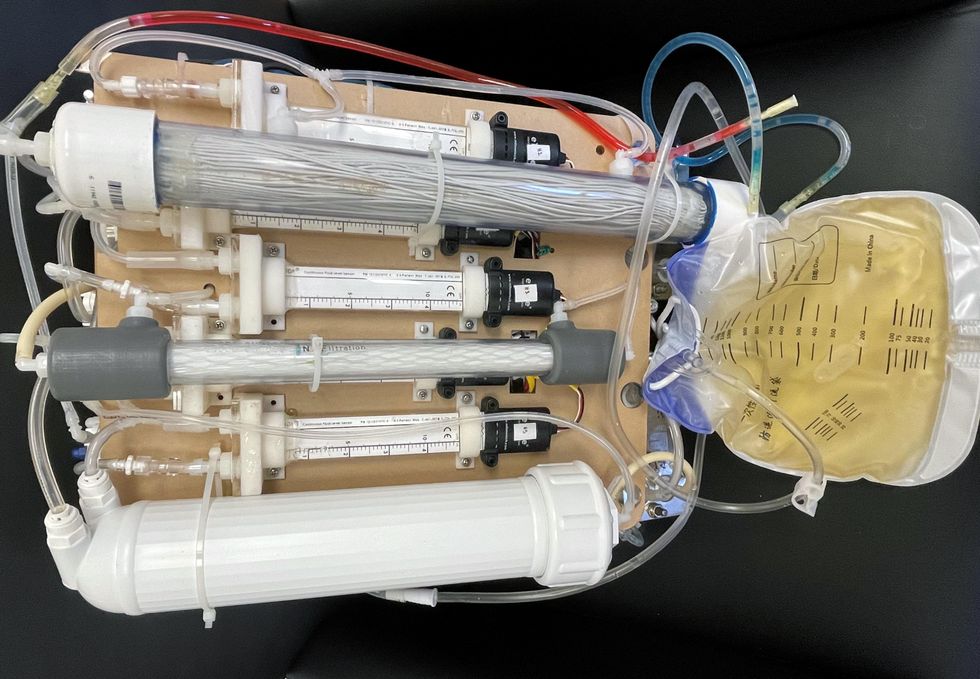
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
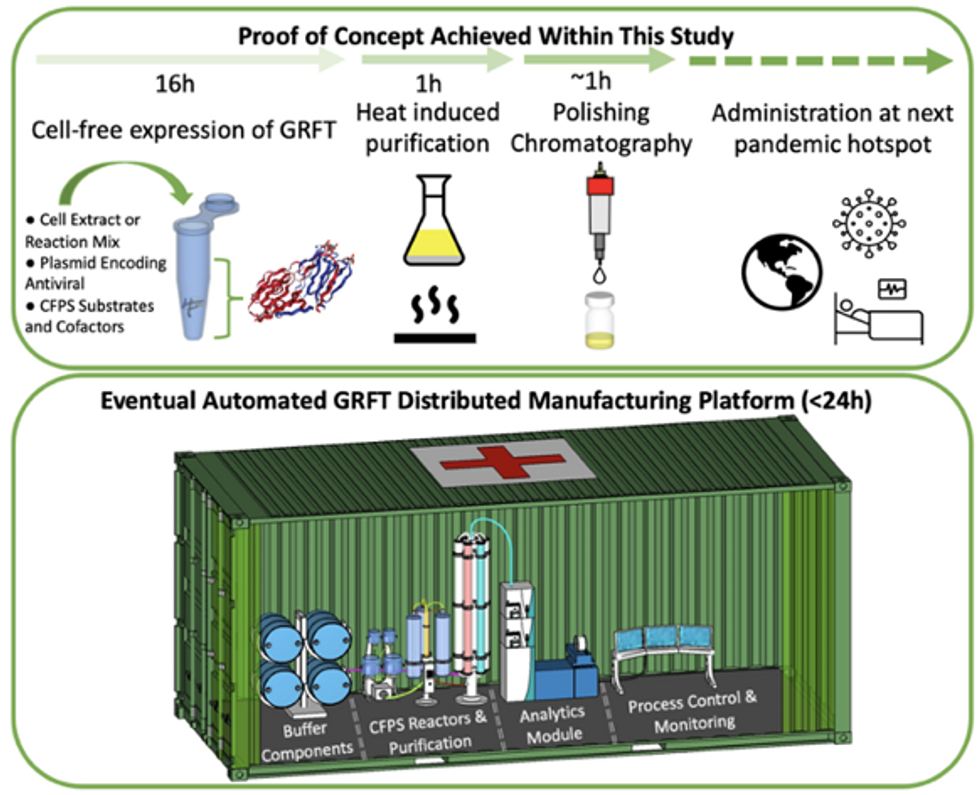
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.