To Save Lives, This Scientist Is Trying to Grow Human Organs Inside of Sheep

A lamb receiving a shot from medical personnel.
More than 114,000 men, women, and children are awaiting organ transplants in the United States. Each day, 22 of them die waiting. To address this shortage, researchers are working hard to grow organs on-demand, using the patient's own cells, to eliminate the need to find a perfectly matched donor.
"The next step is to transplant these cells into a larger animal that will produce an organ that is the right size for a human."
But creating full-size replacement organs in a lab is still decades away. So some scientists are experimenting with the boundaries of nature and life itself: using other mammals to grow human cells. Earlier this year, this line of investigation took a big step forward when scientists announced they had grown sheep embryos that contained human cells.
Dr. Pablo Ross, an associate professor at the University of California, Davis, along with a team of colleagues, introduced human stem cells into the sheep embryos at a very early stage of their development and found that one in every 10,000 cells in the embryo were human. It was an improvement over their prior experiment, using a pig embryo, when they found that one in every 100,000 cells in the pig were human. The resulting chimera, as the embryo is called, is only allowed to develop for 28 days. Leapsmag contributor Caren Chesler recently spoke with Ross about his research. Their interview has been edited and condensed for clarity.
Your goal is to one day grow human organs in animals, for organ transplantation. What does your research entail?
We're transplanting stem cells from a person into an animal embryo, at about day three to five of embryo development.
This concept has already been shown to work between mice and rats. You can grow a mouse pancreas inside a rat, or you can grow a rat pancreas inside a mouse.
For this approach to work for humans, the next step is to transplant these cells into a larger animal that will produce an organ that is the right size for a human. That's why we chose to start some of this preliminary work using pigs and sheep. Adult pigs and adult sheep have organs that are of similar size to an adult human. Pigs and sheep also grow really fast, so they can grow from a single cell at the time of fertilization to human adult size -- about 200 pounds -- in only nine to 10 months. That's better than the average waiting time for an organ transplant.
"You don't want the cells to confer any human characteristics in the animal....Too many cells, that may be a problem, because we do not know what that threshold is."
So how do you get the animal to grow the human organ you want?
First, we need to generate the animal without its own organ. We can generate sheep or pigs that will not grow their own pancreases. Those animals can then be used as hosts for human pancreas generation.
For the approach to work, we need the human stem cells to be able to integrate into the embryo and to contribute to its tissues. What we've been doing with pigs, and more recently, in sheep, is testing different types of stem cells, and introducing them into an early embryo between three to five days of development. We then transfer that embryo to a surrogate female and then harvest the embryos back at day 28 of development, at which point most of the organs are pre-formed.
The human cells will contribute to every organ. But in trying to do that, they will compete with the host organism. Since this is happening inside a pig embryo, which is inside a pig foster mother, the pig cells will win that competition for every organ.
Because you're not putting in enough human cells?
No, because it's a pig environment. Everything is pig. The host, basically, is in control. That's what we see when we do rat mice, or mouse rat: the host always wins the battle.
But we need human cells in the early development -- a few, but not too few -- so that when an organ needs to form, like a pancreas (which develops at around day 25), the pig cells will not respond to that, but if there are human cells in that location, [those human cells] can respond to pancreas formation.
From the work in mice and rats, we know we need some kind of global contribution across multiple tissues -- even a 1% contribution will be sufficient. But if the cells are not there, then they're not going to contribute to that organ. The way we target the specific organ is by removing the competition for that organ.
So if you want it to grow a pancreas, you use an embryo that is not going to grow a pancreas of its own. But you can't control where the other cells go. For instance, you don't want them going to the animal's brain – or its gonads –right?
You don't want the cells to confer any human characteristics in the animal. But even if cells go to the brain, it's not going to confer on the animal human characteristics. A few human cells, even if they're in the brain, won't make it a human brain. Too many cells, that may be a problem, because we do not know what that threshold is.
The objective of our research right now is to look at just 28 days of embryonic development and evaluate what's going on: Are the human cells there? How many? Do they go to the brain? If so, how many? Is this a problem, or is it not a problem? If we find that too many human cells go to the brain, that will probably mean that we wouldn't continue with this approach. At this point, we're not controlling it; we're analyzing it.
"By keeping our research in a very early stage of development, we're not creating a human or a humanoid or anything in between."
What other ethical concerns have arisen?
Conferring human properties to the organism, that is a major concern. I wouldn't like to be involved in that, and so that's what we're trying to assess. By keeping our research in a very early stage of development, we're not creating a human or a humanoid or anything in between.
What specifically sets off the ethical alarms? An animal developing human traits?
Animals developing human characteristics goes beyond what would be considered acceptable. I share that concern. But so far, what we have observed, primarily in rats and mice, is that the host animal dictates development. When you put mouse cells into a rat -- and they're so closely related, sometimes the mouse cells contribute to about 30 percent of the cells in the animal -- the outcome is still a rat. It's the size of a rat. It's the shape of the rat. It has the organ sizes of a rat. Even when the pancreas is fully made out of mouse cells, the pancreas is rat-sized because it grew inside the rat.
This happens even with an organ that is not shared, like a gallbladder, which mice have but rats do not. If you put cells from a mouse into a rat, it never grows a gallbladder. And if you put rat cells into the mouse, the rat cells can end up in the gallbladder even though those rat cells would never have made a gallbladder in a rat.
That means the cell structure is following the directions of the embryo, in terms of how they're going to form and what they're going to make. Based on those observations, if you put human cells into a sheep, we are going to get a sheep with human cells. The organs, the pancreas, in our case, will be the size and shape of the sheep pancreas, but it will be loaded with human cells identical to those of the patient that provided the cells used to generate the stem cells.
But, yeah, if by doing this, the animal acquires the functional or anatomical characteristics associated with a human, it would not be acceptable for me.
So you think these concerns are justified?
Absolutely. They need to be considered. But sometimes by raising these concerns, we prevent technologies from being developed. We need to consider the concerns, but we must evaluate them fully, to determine if they are scientifically justified. Because while we must consider the ethics of doing this, we also need to consider the ethics of not doing it. Every day, 22 people in the US die because they don't receive the organ they need to survive. This shortage is not going to be solved by donations, alone. That's clear. And when people die of old age, their organs are not good anymore.
Since organ transplantation has been so successful, the number of people needing organs has just been growing. The number of organs available has also grown but at a much slower pace. We need to find an alternative, and I think growing the organs in animals is one of those alternatives.
Right now, there's a moratorium on National Institutes of Health funding?
Yes. It's only one agency, but it happens to be the largest biomedical funding source. We have public funding for this work from the California Institute for Regenerative Medicine, and one of my colleagues has funding from the Department of Defense.
"I can say, without NIH funding, it's not going to happen here. It may happen in other places, like China."
Can we put the moratorium in context? How much research in the U.S. is funded by the NIH?
Probably more than 75 percent.
So what kind of impact would lifting that ban have on speeding up possible treatments for those who need a new organ?
Oh, I think it would have a huge impact. The moratorium not only prevents people from seeking funding to advance this area of research, it influences other sources of funding, who think, well, if the NIH isn't doing it, why are we going to do it? It hinders progress.
So with the ban, how long until we can really have organs growing in animals? I've heard five or 10 years.
With or without the ban, I don't think I can give you an accurate estimate.
What we know so far is that human cells don't contribute a lot to the animal embryo. We don't know exactly why. We have a lot of good ideas about things we can test, but we can't move forward right now because we don't have funding -- or we're moving forward but very slowly. We're really just scratching the surface in terms of developing these technologies.
We still need that one major leap in our understanding of how different species interact, and how human cells participate in the development of other species. I cannot predict when we're going to reach that point. I can say, without NIH funding, it's not going to happen here. It may happen in other places, like China, but without NIH funding, it's not going to happen in the U.S.
I think it's important to mention that this is in a very early stage of development and it should not be presented to people who need an organ as something that is possible right now. It's not fair to give false hope to people who are desperate.
So the five to 10 year figure is not realistic.
I think it will take longer than that. If we had a drug right now that we knew could stop heart attacks, it could take five to 10 years just to get it to market. With this, you're talking about a much more complex system. I would say 20 to 25 years. Maybe.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
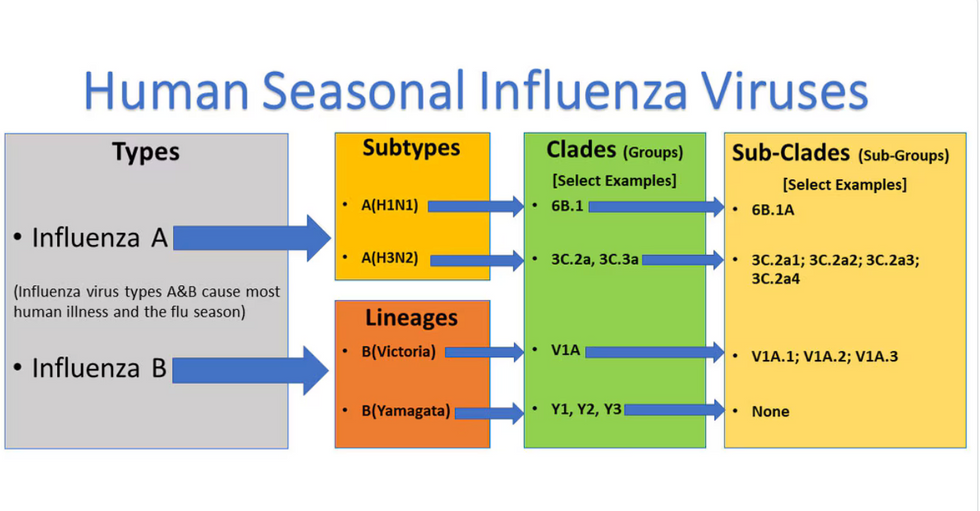
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

