Top Fertility Doctor: Artificially Created Sperm and Eggs "Will Become Normal" One Day
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
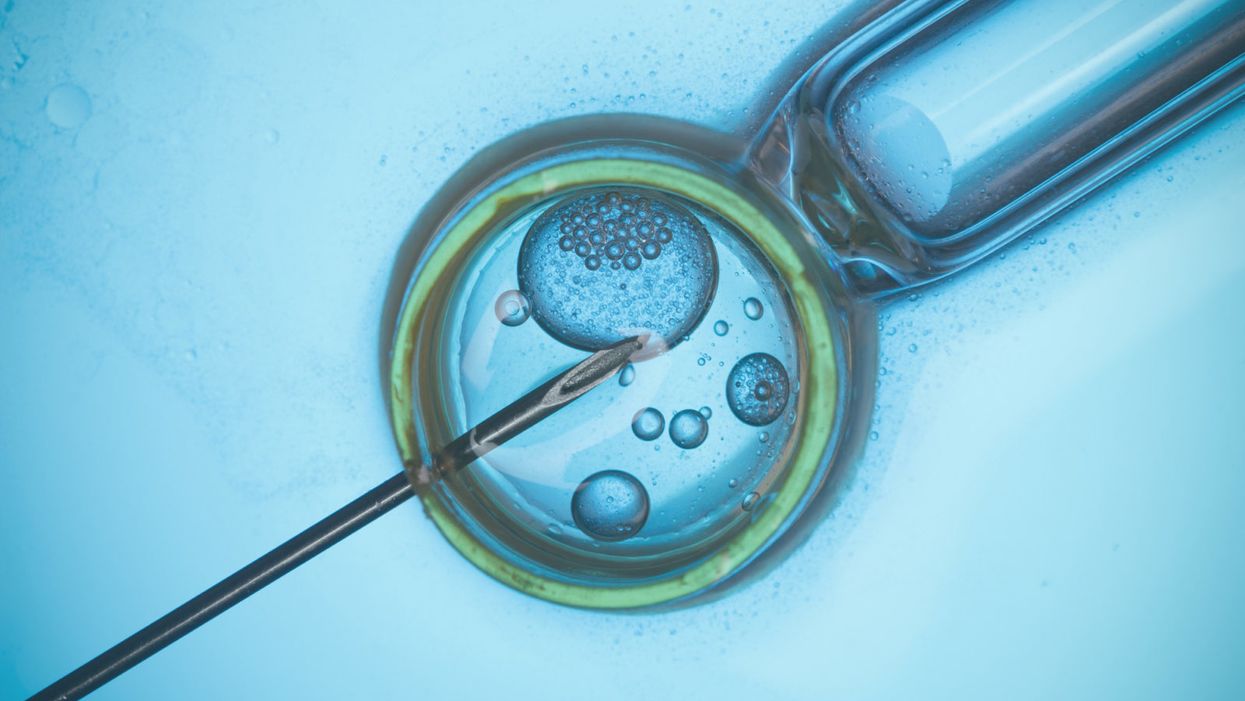
A rendering of in vitro fertilization.
Imagine two men making a baby. Or two women. Or an infertile couple. Or an older woman whose eggs are no longer viable. None of these people could have a baby today without the help of an egg or sperm donor.
Cells scraped from the inside of your cheek could one day be manipulated to become either eggs or sperm.
But in the future, it may be possible for them to reproduce using only their own genetic material, thanks to an emerging technology called IVG, or in vitro gametogenesis.
Researchers are learning how to reprogram adult human cells like skin cells to become lab-created egg and sperm cells, which could then be joined to form an embryo. In other words, cells scraped from the inside of your cheek could one day be manipulated to become either eggs or sperm, no matter your gender or your reproductive fitness.
In 2016, Japanese scientists proved that the concept could be successfully carried out in mice. Now some experts, like Dr. John Zhang, the founder and CEO of New Hope Fertility Center in Manhattan, say it's just "a matter of time" before the method is also made to work in humans.
Such a technological tour de force would upend our most basic assumptions about human reproduction and biology. Combined with techniques like gene editing, these tools could eventually enable prospective parents to have an unprecedented level of choice and control over their children's origins. It's a wildly controversial notion, and an especially timely one now that a Chinese scientist has announced the birth of the first allegedly CRISPR-edited babies. (The claims remain unverified.)
Zhang himself is no stranger to controversy. In 2016, he stunned the world when he announced the birth of a baby conceived using the DNA of three people, a landmark procedure intended to prevent the baby from inheriting a devastating neurological disease. (Zhang went to a clinic in Mexico to carry out the procedure because it is prohibited in the U.S.) Zhang's other achievements to date include helping a 49-year-old woman have a baby using her own eggs and restoring a young woman's fertility through an ovarian tissue transplant surgery.
Zhang recently sat down with our Editor-in-Chief in his New York office overlooking Columbus Circle to discuss the fertility world's latest provocative developments. Here are his top ten insights:
Clearly [gene-editing embryos] will be beneficial to mankind, but it's a matter of how and when the work is done.
1) On a Chinese scientist's claim of creating the first CRISPR-edited babies:
I'm glad that we made a first move toward a clinical application of this technology for mankind. Somebody has to do this. Whether this was a good case or not, there is still time to find out.
Clearly it will be beneficial to mankind, but it's a matter of how and when the work is done. Like any scientific advance, it has to be done in a very responsible way.
Today's response is identical to when the world's first IVF baby was announced in 1978. The major news media didn't take it seriously and thought it was evil, wanted to keep a distance from IVF. Many countries even abandoned IVF, but today you see it is a normal practice. And it took almost 40 years [for the researchers] to win a Nobel Prize.
I think we need more time to understand how this work was done medically, ethically, and let the scientist have the opportunity to present how it was done and let a scientific journal publish the paper. Before these become available, I don't think we should start being upset, scared, or giving harsh criticism.
2) On the international outcry in response to the news:
I feel we are in scientific shock, with many thinking it came too fast, too soon. We all embrace modern technology, but when something really comes along, we fear it. In an old Chinese saying, one of the masters always dreamed of seeing the dragon, and when the dragon really came, he got scared.

Dr. John Zhang, the founder and CEO of New Hope Fertility Center in Manhattan, pictured in his office.
3) On the Western world's perception that Chinese scientists sometimes appear to discount ethics in favor of speedy breakthroughs:
I think this perception is not fair. I don't think China is very casual. It's absolutely not what people think. I don't want people to feel that this case [of CRISPR-edited babies] will mean China has less standards over how human reproduction should be performed. Just because this happened, it doesn't mean in China you can do anything you want.
As far as the regulation of IVF clinics, China is probably the most strictly regulated of any country I know in this world.
4) On China's first public opinion poll gauging attitudes toward gene-edited babies, indicating that more than 60 percent of survey respondents supported using the technology to prevent inherited diseases, but not to enhance traits:
There is a sharp contrast between the general public and the professional world. Being a working health professional and an advocate of scientists working in this field, it is very important to be ethically responsible for what we are doing, but my own feeling is that from time to time we may not take into consideration what the patient needs.
5) On how the three-parent baby is doing today, several years after his birth:
No news is good news.
6) On the potentially game-changing research to develop artificial sperm and eggs:
First of all I think that anything that's technically possible, as long as you are not harmful to other people, to other societies, as long as you do it responsibly, and this is a legitimate desire, I think eventually it will become reality.
My research for now is really to try to overcome the very next obstacle in our field, which is how to let a lady age 44 or older have a baby with her own genetic material.
Practically 99 percent of women over age 43 will never make a baby on their own. And after age 47, we usually don't offer donor egg IVF anymore.
But with improved longevity, and quality of life, the lifespan of females continues to increase. In Japan, the average for females is about 89 years old. So for more than half of your life, you will not be able to produce a baby, which is quite significant in the animal kingdom. In most of the animal kingdom, their reproductive life is very much the same as their life, but then you can argue in the animal kingdom unlike a human being, it doesn't take such a long time for them to contribute to the society because once you know how to hunt and look for food, you're done.
"I think this will become a major ethical debate: whether we should let an older lady have a baby at a very late state of her life."
But humans are different. You need to go to college, get certain skills. It takes 20 years to really bring a human being up to become useful to society. That's why the mom and dad are not supposed to have the same reproductive life equal to their real life.
I think this will become a major ethical debate: whether we should let an older lady have a baby at a very late state of her life and leave the future generation in a very vulnerable situation in which they may lack warm caring, proper guidance, and proper education.
7) On using artificial gametes to grant more reproductive choices to gays and lesbians:
I think it is totally possible to have two sperm make a baby, and two eggs make babies.
If we have two guys, one guy to produce eggs, or two girls, one would have to become sperm. Basically you are creating artificial gametes or converting with gametes from sperm to become egg or egg to become a sperm. Which may not necessarily be very difficult. The key is to be able to do nuclear reprogramming.
So why can two sperm not make offspring now? You get exactly half of your genes from each parent. The genes have their own imprinting that say "made in mom," "made in dad." The two sperm would say "made in dad," "made in dad." If I can erase the "made in dad," and say "made in mom," then these sperm can make offspring.
8) On how close science is to creating artificial gametes for clinical use in pregnancies:
It's very hard to say until we accomplish it. It could be very quick. It could be it takes a long time. I don't want to speculate.
"I think these technologies are the solid foundation just like when we designed the computer -- we never thought a computer would become the iPhone."
9) On whether there should be ethical red lines drawn by authorities or whether the decisions should be left to patients and scientists:
I think we cannot believe a hundred percent in the scientist and the patient but it should not be 100 percent authority. It should be coming from the whole of society.
10) On his expectations for the future:
We are living in a very exciting world. I think that all these technologies can really change the way of mankind and also are not just for baby-making. The research, the experience, the mechanism we learn from these technologies, they will shine some great lights into our long-held dream of being a healthy population that is cancer-free and lives a long life, let's say 120 years.
I think these technologies are the solid foundation just like when we designed the computer -- we never thought a computer would become the iPhone. Imagine making a computer 30 years ago, that this little chip will change your life.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Scientists aim to preserve donkeys, one frozen embryo at a time
In Ethiopia, Samuna’s three donkeys help her transport produce to market and to collect the water essential to her family, neighbours and livestock. Donkeys are more endangered than people realize, experts say.
Every day for a week in 2022, Andres Gambini, a veterinarian and senior lecturer in animal science at the University of Queensland in Australia, walked into his lab—and headed straight to the video camera. Trained on an array of about 50 donkey embryos, all created by Gambini’s manual in vitro fertilization, or IVF, the camera kept an eye on their developmental progress. To eventually create a viable embryo that could be implanted into a female donkey, the embryos’ cells had to keep dividing, first in two, then in four and so on.
But the embryos weren’t cooperating. Some would start splitting up only to stop a day or two later, and others wouldn’t start at all. Every day he came in, Gambini saw fewer and fewer dividing embryos, so he was losing faith in the effort. “You see many failed attempts and get disappointed,” he says.
Gambini and his team, a group of Argentinian and Spanish researchers, were working to create these embryos because many donkey populations around the world are declining. It may sound counterintuitive that domesticated animals may need preservation, but out of 28 European donkey breeds, 20 are endangered and seven are in critical status. It is partly because of the inbreeding that happened over the course of many years and partly because in today’s Western world donkeys aren’t really used anymore.
“That's the reason why some breeds begin to disappear because humans were not really interested in having that specific breed anymore,” Gambini says. Nonetheless, in Africa, India and Latin America millions of rural families still rely on these hardy creatures for agriculture and transportation. And the only two wild donkey species—Equus africanus in Africa and Equus hemionus in Asia—are also dwindling, due to losing their habitats to human activities, diseases and slow reproduction rates. Gambini’s team wanted to create a way to preserve the animals for the future. “Donkeys are more endangered than people realize,” he says.
There’s much more to donkeys' trouble though. For the past 20 or so years, they have been facing a huge existential threat due to their hide gelatin, a compound derived from their skins by soaking and stewing. In Chinese traditional medicine, the compound, called ejiao, is believed to have a medicinal value, so it’s used in skin creams, added to food and taken in capsules. Centuries ago, ejiao was a very expensive luxury product available only for the emperor and his household. That changed in the 1990s when the Chinese economy boomed, and many people were suddenly able to afford it. “It went from a very elite product to a very popular product,” says Janneke Merkx, a campaign manager at The Donkey Sanctuary, a United Kingdom-based nonprofit organization that keeps tabs on the animals’ welfare worldwide. “It is a status symbol for gift giving.”
Having evolved in the harsh and arid mountainous terrains where food and water were scarce, donkeys are extremely adaptable and hardy. But the Donkey Sanctuary documented cases in which an entire village had their animals disappear overnight, finding them killed and skinned outside their settlement.
The Chinese donkey population was quickly decimated. Unlike many other farm animals, donkeys are finicky breeders. When stressed and unhappy, they don’t procreate, so growing them in large industrial settings isn’t possible. “Donkeys are notoriously slow breeders and really very difficult to farm,” says Merkx. “They are not the same as other livestock like sheep and pigs and cattle.” Within years the, the donkey numbers in China dropped precipitously. “China used to have the largest donkey population in the world in the 1990s. They had 11 million donkeys, and it's now down to less than 3 million, and they just can't keep up with the demand.”
To keep the ejiao conveyor going, some producers turned to the illegal wildlife trade. Poachers began to steal and slaughter donkeys from rural villages in Africa. The Donkey Sanctuary documented cases in which an entire village had their animals disappear overnight, finding them killed and skinned outside their settlement. Exactly how many creatures were lost to the skin trade to-date isn’t possible to calculate, says Faith Burden, the Donkey Sanctuary’s director of equine operations. Traditionally a poor people’s beast of burden, donkey counts are hard to keep track of. “When an animal doesn't produce meat, milk or eggs or whatever edible product, they're often less likely to be acknowledged in a government population census,” Burden says. “So reliable statistics are hard to come by.” The nonprofit estimates that about 4.8 million are slaughtered annually.
During their six to seven thousand years of domestication, donkeys rarely got the full appreciation for their services. They are often compared to horses, which doesn’t do them justice. They’re entirely different animals, Burden says. Built for speed, horses respond to predators and other dangers by running as fast as they can. Donkeys, which originate from the rocky, mountainous regions of Africa where running is dangerous, react to threats by freezing and assessing the situation for the best response. “Those so-called stubborn donkeys that won’t move as you want, they are actually thinking ‘what’s the best approach,’” Burden says. They may even choose to fight the predators rather than flee, she adds. “In some parts of the world, people use them as guard animals against things like coyotes and wolves.”
Scientists believe that domestic donkeys take their origin from Equus africanus or African wild ass, originally roaming where Kenya, Ethiopia and Eritrea are today. Having evolved in the harsh and arid mountainous terrains where food and water were scarce, they are extremely adaptable and hardy. Research finds that they can go without water for 72 hours and then drink their fill without any negative consequences. Their big jaws let them chew tough desert shrubs, which horses can’t exist on. Their large ears help dissipate heat. Their little upright hooves are a perfect fit for the uneven rocky or other dangerous grounds. Accustomed to the mountain desert climate with hot days and cold nights, they don’t mind temperature flux.
“The donkey is the most supremely adapted animal to deal with hostile conditions,” Burden says. “They can survive on much lower nutritional quality food than a cow, sheep or horse. That’s why communities living in some of the most inhospitable places will often have donkeys with them.” And that’s why losing a donkey to an illegal skin trade can devastate a family in places like Eritrea. Suddenly everything from water to firewood to produce must be carried by family members—and often women.

Workers unloading donkeys at the Shinyanga slaughterhouse in Tanzania. Fearing a future in which donkeys go extinct, scientists have found ways to cryopreserve a donkey embryo in liquid nitrogen.
TAHUCHA
One can imagine a time when worldwide donkey populations may dwindle to the point that they would need to be restored. That includes their genetic variability too. That’s where the frozen embryos may come in handy. We may be able to use them to increase the genetic variability of donkeys, which will be especially important if they get closer to extinction, Gambini says. His team had already created frozen embryos for horses and zebras, an idea similar to a seed bank. “We call this concept the Frozen Zoo.”
Creating donkey embryos proved much harder than those of zebras and horses. To improve chances of fertilization, Gambini used the intracytoplasmic sperm injection or ICSI, in which he employed a tiny needle called a micropipette to inject a donkey sperm into an egg. That was a step above the traditional IVF method, in which the egg and a sperm are left floating in a test tube together. The injection took, but during the incubating week, one after the other, the embryos stopped dividing. Finally, on day seven, Gambini finally spotted the exact sight he was hoping to see. One of the embryos developed into a burgeoning ball of cells.
“That stage is called a blastocyst,” Gambini says. The clump of cells had a lot of fluids mixed within them, which indicated that they were finally developing into a viable embryo. “When we see a blastocyst, we know we can transfer that into a female.” He was so excited he immediately called all his collaborators to tell them the good news, which they later published in the journal of Theriogenology.
The one and only embryo to reach that stage, the blastocyst was cryopreserved in liquid nitrogen. The team is waiting for the next breeding season to see if a female donkey may carry it to term and give birth to a healthy foal. Gambini’s team is hoping to polish the process and create more embryos. “It’s our weapon in the conservation ass-enal,” he says.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Too much of this ingredient leads to autoimmune diseases, new research shows. Here's how to cut back.
Scientists are looking at how salt affects our cells, and they're finding more reasons to avoid htoo much of it.
For more than a century, doctors have warned that too much salt in your diet can lead to high blood pressure, heart disease and stroke - and many of the reasons for these effects are well known. But recently scientists have been looking deeper, into the cellular level, and they are finding additional reasons to minimize sodium intake; it is bad for immune cells, creating patterns of gene expression and activity seen in a variety of autoimmune diseases such as multiple sclerosis, lupus, rheumatoid arthritis, and type-1 diabetes.
Salt is a major part of the ocean from which life evolved on this planet. We carry that legacy in our blood, which tastes salty. It is an important element for conducting electrical signals along nerves and balancing water and metabolites transported throughout our bodies. We need to consume about 500 milligrams of salt each day to maintain these functions, more with exercise and heavy sweating as that is a major way the body loses salt. The problem is that most Americans eating a modern western diet consume about 3400 milligrams, 1.5 teaspoons per day.
Evidence has been accumulating over the last few years that elevated levels of sodium can be harmful to at least some types of immune cells. The first signal came in monocytes, which are immune cells that travel to various tissues in the body, where some of them turn into macrophages, a subset of white blood cells that can directly kill microorganisms and make chemical signals that bring other types of immune cells into play.
Two years ago, Dominik N. Müller from the Max-Delbrueck-Center in Berlin, Germany and Markus Kleinewietfeld, an immunologist at Hasselt University in Belgium, ran a study where they fed people pizza and then measured their immune cell function. “We saw that in any monocytes, metabolic function was down, even after a single salty meal,” Kleinewietfeld says. It seemed to be the cellular equivalent of the sluggish feeling we get after eating too much. The cells were able to recover but more research is needed to answer questions about what dose of sodium causes impairment, how long the damage lasts, and whether there is a cumulative effect of salt toxicity.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations.
The latest series of experiments focused on a type of T cell called T regulatory cells, or Tregs. Most T cells release inflammatory mediators to fight pathogens and, once that job is done, Tregs come along to calm down their hyperactive brethren. Failure to do so can result in continued inflammation and possibly autoimmune diseases.
In the lab, Kleinewietfeld and his large team of international collaborators saw that high levels of sodium had a huge effect on Tregs, upregulating 1250 genes and downregulating an additional 1380 genes so that they looked similar to patterns of gene expression seen in autoimmune diseases.
Digging deeper, they found that sodium affected mitochondria, the tiny organelles inside of cells that produce much of its energy. The sodium was interfering with how the mitochondria use oxygen, which resulted in increased levels of an unstable form of oxygen that can damage cell function. The researchers injected those damaged Tregs into mice and found that they impaired the animals' immune function, allowing the inflammation to continue rather than shutting it down.
That finding dovetailed nicely with a 2019 paper in Nature from Navdeep Chandel's lab at Northwestern University, which showed in mice that inhibiting the mitochondrial use of oxygen reduced the ability of Tregs to regulate other T cells. “Mitochondria were controlling directly the immunosuppressive program, they were this master regulator tuning the right amount of genes to give you proper immunosuppression,” Chandel said. “And if you lose that function, then you get autoimmunity.”
Kleinewietfeld's team studied the Treg cells of humans and found that sodium can similarly decrease mitochondrial use of oxygen and immunosuppressive activity. “I would have never predicted that myself,” Chandel says, but now researchers can look at the mitochondria of patients with autoimmune disease and see if their gene expression also changes under high salt conditions. He sees the link between the patterns of gene expression in Tregs generated by high salt exposure and those patterns seen in autoimmune diseases, but he is cautious about claiming a causal effect.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations. He says a high salt diet could also have an indirect effect on immune function through the way it affects the gut microbiome and the molecules made by microbes when they break down food. But the research results are too preliminary to say that for sure, much less parse out the role of salt compared with other possible factors. “It is still an exciting journey to try to understand this field,” he says.
Additionally, it is difficult to say precisely how this research in animals and human cell cultures will translate into a whole human body. Individual differences in genetics can affect how the body absorbs, transports, and gets rid of sodium, such that some people are more sensitive to salt than are others.
So how should people apply these research findings to daily life?
Salt is obvious when we sprinkle it on at the table or eat tasty things like potato chips, but we may be unaware of sodium hidden in packaged foods. That's because salt is an easy and cheap way to boost the flavor of foods. And if we do read the labeled salt content on a package, we focus on the number for a single serving, but then eat more than that.
Last September, the U.S. Food and Drug Administration (FDA) began a process to update labels on the content of food, including what is meant by the word “healthy” and how food manufacturers can use the term. Many in the food industry are resisting those proposed changes.
Chandel cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker.
Until labels are updated, it would be prudent to try to reduce sodium intake by cutting down on packaged foods while making your own food at home, where you know just how much salt has been added. The Mayo Clinic offers guidance on how to become more aware of the sodium in your diet and eat less of it.
Chandel thinks many people will struggle with minimizing salt in their diets. It’s similar to the challenge of eating less sugar, in that the body craves both, and it is difficult to fight that. He cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker. “Dietary antioxidants have failed in just about every clinical trial, yet the public continues to take them,” Chandel says. But he is optimistic that research will lead us to a better understanding of how Tregs function, and uncover new targets for treating autoimmune diseases.

