New approach to brain health is sparking memories
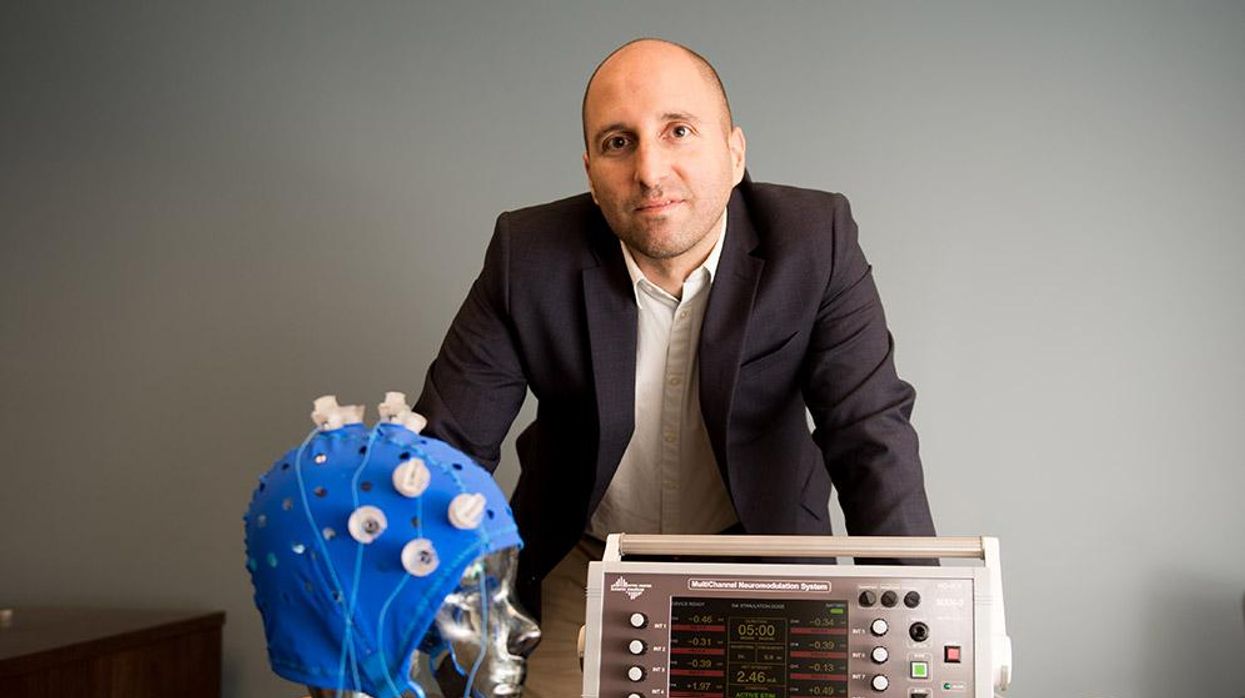
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”
The future of non-hormonal birth control: Antibodies can stop sperm in their tracks
Many women want non-hormonal birth control. A 22-year-old's findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
Unwanted pregnancy can now be added to the list of preventions that antibodies may be fighting in the near future. For decades, really since the 1980s, engineered monoclonal antibodies have been knocking out invading germs — preventing everything from cancer to COVID. Sperm, which have some of the same properties as germs, may be next.
Not only is there an unmet need on the market for alternatives to hormonal contraceptives, the genesis for the original research was personal for the then 22-year-old scientist who led it. Her findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
The genesis
It’s Suruchi Shrestha’s research — published in Science Translational Medicine in August 2021 and conducted as part of her dissertation while she was a graduate student at the University of North Carolina at Chapel Hill — that could change the future of contraception for many women worldwide. According to a Guttmacher Institute report, in the U.S. alone, there were 46 million sexually active women of reproductive age (15–49) who did not want to get pregnant in 2018. With the overturning of Roe v. Wade last year, Shrestha’s research could, indeed, be life changing for millions of American women and their families.
Now a scientist with NextVivo, Shrestha is not directly involved in the development of the contraceptive that is based on her research. But, back in 2016 when she was going through her own problems with hormonal contraceptives, she “was very personally invested” in her research project, Shrestha says. She was coping with a long list of negative effects from an implanted hormonal IUD. According to the Mayo Clinic, those can include severe pelvic pain, headaches, acute acne, breast tenderness, irregular bleeding and mood swings. After a year, she had the IUD removed, but it took another full year before all the side effects finally subsided; she also watched her sister suffer the “same tribulations” after trying a hormonal IUD, she says.
For contraceptive use either daily or monthly, Shrestha says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
Shrestha unshelved antibody research that had been sitting idle for decades. It was in the late 80s that scientists in Japan first tried to develop anti-sperm antibodies for contraceptive use. But, 35 years ago, “Antibody production had not been streamlined as it is now, so antibodies were very expensive,” Shrestha explains. So, they shifted away from birth control, opting to focus on developing antibodies for vaccines.
Over the course of the last three decades, different teams of researchers have been working to make the antibody more effective, bringing the cost down, though it’s still expensive, according to Shrestha. For contraceptive use either daily or monthly, she says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
The problem
The problem with contraceptives for women, Shrestha says, is that all but a few of them are hormone-based or have other negative side effects. In fact, some studies and reports show that millions of women risk unintended pregnancy because of medical contraindications with hormone-based contraceptives or to avoid the risks and side effects. While there are about a dozen contraceptive choices for women, there are two for men: the condom, considered 98% effective if used correctly, and vasectomy, 99% effective. Neither of these choices are hormone-based.
On the non-hormonal side for women, there is the diaphragm which is considered only 87 percent effective. It works better with the addition of spermicides — Nonoxynol-9, or N-9 — however, they are detergents; they not only kill the sperm, they also erode the vaginal epithelium. And, there’s the non-hormonal IUD which is 99% effective. However, the IUD needs to be inserted by a medical professional, and it has a number of negative side effects, including painful cramping at a higher frequency and extremely heavy or “abnormal” and unpredictable menstrual flows.
The hormonal version of the IUD, also considered 99% effective, is the one Shrestha used which caused her two years of pain. Of course, there’s the pill, which needs to be taken daily, and the birth control ring which is worn 24/7. Both cause side effects similar to the other hormonal contraceptives on the market. The ring is considered 93% effective mostly because of user error; the pill is considered 99% effective if taken correctly.
“That’s where we saw this opening or gap for women. We want a safe, non-hormonal contraceptive,” Shrestha says. Compounding the lack of good choices, is poor access to quality sex education and family planning information, according to the non-profit Urban Institute. A focus group survey suggested that the sex education women received “often lacked substance, leaving them feeling unprepared to make smart decisions about their sexual health and safety,” wrote the authors of the Urban Institute report. In fact, nearly half (45%, or 2.8 million) of the pregnancies that occur each year in the US are unintended, reports the Guttmacher Institute. Globally the numbers are similar. According to a new report by the United Nations, each year there are 121 million unintended pregnancies, worldwide.
The science
The early work on antibodies as a contraceptive had been inspired by women with infertility. It turns out that 9 to 12 percent of women who are treated for infertility have antibodies that develop naturally and work against sperm. Shrestha was encouraged that the antibodies were specific to the target — sperm — and therefore “very safe to use in women.” She aimed to make the antibodies more stable, more effective and less expensive so they could be more easily manufactured.
Since antibodies tend to stick to things that you tell them to stick to, the idea was, basically, to engineer antibodies to stick to sperm so they would stop swimming. Shrestha and her colleagues took the binding arm of an antibody that they’d isolated from an infertile woman. Then, targeting a unique surface antigen present on human sperm, they engineered a panel of antibodies with as many as six to 10 binding arms — “almost like tongs with prongs on the tongs, that bind the sperm,” explains Shrestha. “We decided to add those grabbers on top of it, behind it. So it went from having two prongs to almost 10. And the whole goal was to have so many arms binding the sperm that it clumps it” into a “dollop,” explains Shrestha, who earned a patent on her research.

Suruchi Shrestha works in the lab with a colleague. In 2016, her research on antibodies for birth control was inspired by her own experience with side effects from an implanted hormonal IUD.
UNC - Chapel Hill
The sperm stays right where it met the antibody, never reaching the egg for fertilization. Eventually, and naturally, “Our vaginal system will just flush it out,” Shrestha explains.
“She showed in her early studies that [she] definitely got the sperm immotile, so they didn't move. And that was a really promising start,” says Jasmine Edelstein, a scientist with an expertise in antibody engineering who was not involved in this research. Shrestha’s team at UNC reproduced the effect in the sheep, notes Edelstein, who works at the startup Be Biopharma. In fact, Shrestha’s anti-sperm antibodies that caused the sperm to agglutinate, or clump together, were 99.9% effective when delivered topically to the sheep’s reproductive tracts.
The future
Going forward, Shrestha thinks the ideal approach would be delivering the antibodies through a vaginal ring. “We want to use it at the source of the spark,” Shrestha says, as opposed to less direct methods, such as taking a pill. The ring would dissolve after one month, she explains, “and then you get another one.”
Engineered to have a long shelf life, the anti-sperm antibody ring could be purchased without a prescription, and women could insert it themselves, without a doctor. “That's our hope, so that it is accessible,” Shrestha says. “Anybody can just go and grab it and not worry about pregnancy or unintended pregnancy.”
Her patented research has been licensed by several biotech companies for clinical trials. A number of Shrestha’s co-authors, including her lab advisor, Sam Lai, have launched a company, Mucommune, to continue developing the contraceptives based on these antibodies.
And, results from a small clinical trial run by researchers at Boston University Chobanian & Avedisian School of Medicine show that a dissolvable vaginal film with antibodies was safe when tested on healthy women of reproductive age. That same group of researchers last year received a $7.2 million grant from the National Institute of Health for further research on monoclonal antibody-based contraceptives, which have also been shown to block transmission of viruses, like HIV.
“As the costs come down, this becomes a more realistic option potentially for women,” says Edelstein. “The impact could be tremendous.”
This article was first published by Leaps.org in December, 2022. It has been lightly edited with updates for timeliness.
Researchers probe extreme gene therapy for severe alcoholism
When all traditional therapeutic approaches fail for alcohol abuse disorder, a radical gene therapy might be something to try in the future.
Story by Freethink
A single shot — a gene therapy injected into the brain — dramatically reduced alcohol consumption in monkeys that previously drank heavily. If the therapy is safe and effective in people, it might one day be a permanent treatment for alcoholism for people with no other options.
The challenge: Alcohol use disorder (AUD) means a person has trouble controlling their alcohol consumption, even when it is negatively affecting their life, job, or health.
In the U.S., more than 10 percent of people over the age of 12 are estimated to have AUD, and while medications, counseling, or sheer willpower can help some stop drinking, staying sober can be a huge struggle — an estimated 40-60 percent of people relapse at least once.
A team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
According to the CDC, more than 140,000 Americans are dying each year from alcohol-related causes, and the rate of deaths has been rising for years, especially during the pandemic.
The idea: For occasional drinkers, alcohol causes the brain to release more dopamine, a chemical that makes you feel good. Chronic alcohol use, however, causes the brain to produce, and process, less dopamine, and this persistent dopamine deficit has been linked to alcohol relapse.
There is currently no way to reverse the changes in the brain brought about by AUD, but a team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
To find out, they tested it in heavy-drinking monkeys — and the animals’ alcohol consumption dropped by 90% over the course of a year.
How it works: The treatment centers on the protein GDNF (“glial cell line-derived neurotrophic factor”), which supports the survival of certain neurons, including ones linked to dopamine.
For the new study, a harmless virus was used to deliver the gene that codes for GDNF into the brains of four monkeys that, when they had the option, drank heavily — the amount of ethanol-infused water they consumed would be equivalent to a person having nine drinks per day.
“We targeted the cell bodies that produce dopamine with this gene to increase dopamine synthesis, thereby replenishing or restoring what chronic drinking has taken away,” said co-lead researcher Kathleen Grant.
To serve as controls, another four heavy-drinking monkeys underwent the same procedure, but with a saline solution delivered instead of the gene therapy.
The results: All of the monkeys had their access to alcohol removed for two months following the surgery. When it was then reintroduced for four weeks, the heavy drinkers consumed 50 percent less compared to the control group.
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
The researchers then took the alcohol away for another four weeks, before giving it back for four. They repeated this cycle for a year, and by the end of it, the treated monkeys’ consumption had fallen by more than 90 percent compared to the controls.
“Drinking went down to almost zero,” said Grant. “For months on end, these animals would choose to drink water and just avoid drinking alcohol altogether. They decreased their drinking to the point that it was so low we didn’t record a blood-alcohol level.”
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
Looking ahead: Dopamine is involved in a lot more than addiction, so more research is needed to not only see if the results translate to people but whether the gene therapy leads to any unwanted changes to mood or behavior.
Because the therapy requires invasive brain surgery and is likely irreversible, it’s unlikely to ever become a common treatment for alcoholism — but it could one day be the only thing standing between people with severe AUD and death.
“[The treatment] would be most appropriate for people who have already shown that all our normal therapeutic approaches do not work for them,” said Grant. “They are likely to create severe harm or kill themselves or others due to their drinking.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.


