New drug for schizophrenia could meet desperate need for better treatments

The field of treating schizophrenia with drugs has been stuck in a long drought but, this month, a late-stage clinical trial found a new drug called KarXT could treat a range of symptoms.
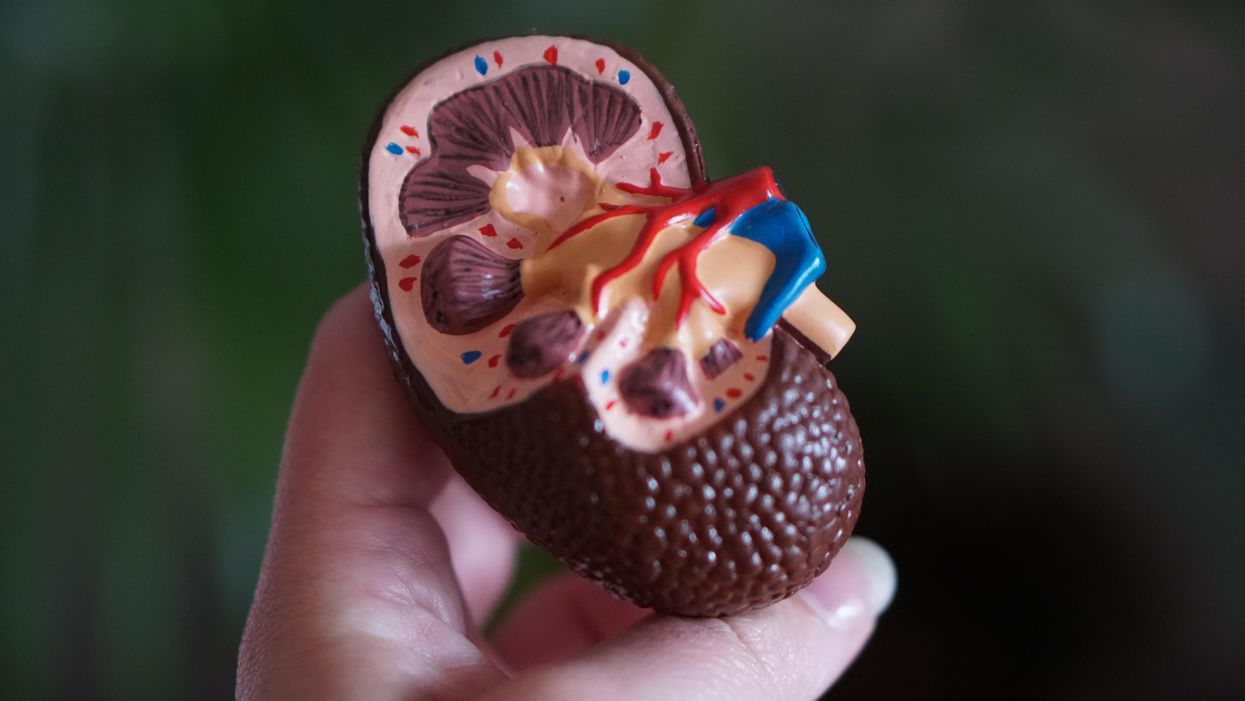
Schizophrenia is a debilitating mental health condition that affects around 24 million people worldwide. Patients experience hallucinations and delusions when they develop schizophrenia, with experts referring to these new thoughts and behaviors as positive symptoms. They also suffer from negative symptoms in which they lose important functions, suffering from dulled emotions, lack of purpose and social withdrawal.
Currently available drugs can control only a portion of these symptoms but, on August 8th, Karuna Therapeutics announced its completion of a phase 3 clinical trial that found a new drug called KarXT could treat both positive and negative symptoms of schizophrenia. It could mean substantial progress against a problem that has stymied scientists for decades.
A long-standing problem
Since the 1950s, antipsychotics have been used to treat schizophrenia. People who suffer from it are thought to have too much of a brain chemical called dopamine, and antipsychotics work by blocking dopamine receptors in the brain. They can be effective in treating positive symptoms but have little impact on the negative ones, which can be devastating for a patient’s quality of life, making it difficult to maintain employment and have successful relationships. About 30 percent of schizophrenia patients don't actually respond to antipsychotics at all. Current drugs can also have adverse side effects including elevated cholesterol, high blood pressure, diabetes and movements that patients cannot control.
The recent clinical trial heralds a new treatment approach. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” says Andrew Miller, COO of Karuna.
Scientists have been looking to develop alternatives. However, “the field of drug treatment of schizophrenia is currently in the doldrums,” says Peter McKenna, a senior researcher at FIDMAG Research Foundation in Spain which specialises in mental health.
In the 2000s there was a major push to target a brain receptor for a chemical called glutamate. Evidence suggested that this receptor is abnormal in the brains of schizophrenia patients, but attempts to try glutamate failed in clinical trials.
After that, many pharmaceutical companies dropped out of the race for a more useful treatment. But some companies continued to search, such as Karuna Therapeutics, led by founder and Chief Operating Officer Andrew Miller and CEO Steve Paul. The recent clinical trial suggests their persistence has led to an important breakthrough with their drug, KarXT. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” Miller says.
How it works
Neurotransmitters are chemical messengers that pass signals between neurons. To work effectively, neurotransmitters need a receptor to bind to. A neurotransmitter called acetylcholine seems to be especially important in schizophrenia. It interacts with sites called muscarinic receptors, which are involved in the network of nerves that calm your body after a stressful event. Post mortem studies in people with schizophrenia have shown that two muscarinic receptors in the brain, the M1 and M4 receptors, are activated at unusually low levels because they don’t receive enough signals from acetylcholine.
The M4 receptor appears to play a role in psychosis. The M1 receptor is also associated with psychosis but is primarily thought to be involved in cognition. KarXT, taken orally, works by activating both of these receptors to signal properly. It is this twofold action that seems to explain its effectiveness. “[The drug’s] design enables the preferential stimulation of these muscarinic receptors in the brain,” Miller says.
How it developed
It all started in the early 1990s when Paul was at pharmaceutical company Eli Lilly. He discovered that Xanomeline, the drug they were testing on Alzheimer's patients, had antipsychotic effects. It worked by stimulating M1 and M4 receptors, so he and his colleagues decided to test Xanomeline on schizophrenia patients, supported by research on the connection between muscarinic receptors and psychosis. They found that Xanomeline reduced both positive and negative symptoms.
Unfortunately, it also caused significant side effects. The problem was that stimulating the M1 and M4 receptors in the brain also stimulated muscarinic receptors in the body that led to severe vomiting, diarrhea and even the temporary loss of consciousness.
In the end, Eli Lilly discontinued the clinical trials for the drug, but Miller set up Karuna Therapeutics to develop a solution. “I was determined to find a way to harness the therapeutic benefit demonstrated in studies of Xanomeline, while eliminating side effects that limited its development,” Miller says.
He analysed over 7,000 possible ways of mixing Xanomeline with other agents before settling on KarXT. It combines Xanomeline with a drug called Trospium Chloride, which blocks muscarinic receptors in the body – taking care of the side effects such as vomiting – but leaves them unblocked in the brain. Paul was so excited by Miller’s progress that he joined Karuna after leaving Eli Lilly and founding two previous startups.
“It's a very important approach,” says Rick Adams, Future Leaders Fellow in the Institute of Cognitive Neuroscience and Centre for Medical Image Computing at University College London. “We are in desperate need of alternative drug targets and this target is one of the best. There are other alternative targets, but not many are as close to being successful as the muscarinic receptor drug.”
Clinical Trial
Following a successful phase 2 clinical trial in 2019, the most recent trial involved 126 patients who were given KarXT, and 126 who were given a placebo. Compared to the placebo, patients taking KarXT had a significant 9.6 point reduction in the positive and negative syndrome scale (PANSS), the standard for rating schizophrenic symptoms.
KarXT also led to statistically significant declines in positive and negative symptoms compared to the placebo. “The results suggest that KarXT could be a potentially game-changing option in the management of both positive and negative symptoms of schizophrenia,” Miller says.
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, is optimistic about the side effects but highlights the need for more safety trials.
McKenna, the researcher at FIDMAG Foundation, agrees about the drug’s potential. “The new [phase 3] study is positive,” he says. “It is reassuring that one is not dealing with a drug that works in one trial and then inexplicably fails in the next one.”
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, said the drug is an unprecedented step forward. “KarXT is one of the first drugs with a novel mechanism of action to show promise in clinical trials.”
Even though the drug blocks muscarine receptors in the body, some patients still suffered from adverse side effects like vomiting, dizziness and diarrhea. But in general, these effects were mild to moderate, especially compared to dopamine-blocking antipsychotics or Xanomeline on its own.
McCutcheon is optimistic about the side effects but highlights the need for more safety trials. “The trial results suggest that gastrointestinal side effects appear to be manageable,” he says. “We know, however, from previous antipsychotic drugs that the full picture regarding the extent of side effects can sometimes take longer to become apparent to clinicians and patients. Careful ongoing assessment during a longer period of treatment will therefore be important.”
The Future
The team is currently conducting three other trials to evaluate the efficacy and long-term safety of KarXT. Their goal is to receive FDA approval next year.
Karuna is also conducting trials to evaluate the effectiveness of KarXT in treating psychosis in patients suffering from Alzheimer’s.
The big hope is that they will soon be able to provide a radically different drug to help many patients with schizophrenia. “We are another step closer to potentially providing the first new class of medicine in more than 50 years to the millions of people worldwide living with schizophrenia,” says Miller.
Scientists at Baylor College of Medicine developed a vaccine called Corbevax that, unlike mRNA vaccines, can be mass produced using technology already in place in low- and middle-income countries. It's now being administered in India to children aged 12-14.
When the COVID-19 pandemic began invading the world in late 2019, Peter Hotez and Maria Elena Bottazzi set out to create a low-cost vaccine that would help inoculate populations in low- and middle-income countries. The scientists, with their prior experience of developing inexpensive vaccines for the world’s poor, had anticipated that the global rollout of Covid-19 jabs would be marked with several inequities. They wanted to create a patent-free vaccine to bridge this gap, but the U.S. government did not seem impressed, forcing the researchers to turn to private philanthropies for funds.
Hotez and Bottazzi, both scientists at the Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine, raised about $9 million in private funds. Meanwhile, the U.S. government’s contribution stood at $400,000.
“That was a very tough time early on in the pandemic, you know, trying to do the work and raise the money for it at the same time,” says Hotez, who was nominated in February for a Nobel Peace Prize with Bottazzi for their COVID-19 vaccine. He adds that at the beginning of the pandemic, governments emphasized speed, innovation and rapidly immunizing populations in North America and Europe with little consideration for poorer countries. “We knew this [vaccine] was going to be the answer to global vaccine inequality, but I just wish the policymakers had felt the same,” says Hotez.
Over the past two years, the world has witnessed 488 million COVID-19 infections and over 61 million deaths. Over 11 billion vaccine doses have been administered worldwide; however, the global rollout of COVID-19 vaccines is marked with alarming socio-economic inequities. For instance, 72 percent of the population in high-income countries has received at least one dose of the vaccine, whereas the number stands at 15 percent in low-income countries.
This inequity is worsening vulnerabilities across the world, says Lawrence Young, a virologist and co-lead of the Warwick Health Global Research Priority at the UK-based University of Warwick. “As long as the virus continues to spread and replicate, particularly in populations who are under-vaccinated, it will throw up new variants and these will remain a continual threat even to those countries with high rates of vaccination,” says Young, “Therefore, it is in all our interests to ensure that vaccines are distributed equitably across the world.”
“When your house is on fire, you don't call the patent attorney,” says Hotez. “We wanted to be the fire department.”
The vaccine developed by Hotez and Bottazzi recently received emergency use authorisation in India, which plans to manufacture 100 million doses every month. Dubbed ‘Corbevax’ by its Indian maker, Biological E Limited, the vaccine is now being administered in India to children aged 12-14. The patent-free arrangement means that other low- and middle-income countries could also produce and distribute the vaccine locally.
“When your house is on fire, you don't call the patent attorney, you call the fire department,” says Hotez, commenting on the intellectual property rights waiver. “We wanted to be the fire department.”
The Inequity
Vaccine equity simply means that all people, irrespective of their location, should have equal access to vaccines. However, data suggests that the global COVID-19 vaccine rollout has favoured those in richer countries. For instance, high-income countries like the UAE, Portugal, Chile, Singapore, Australia, Malta, Hong Kong and Canada have partially vaccinated over 85 percent of their populations. This percentage in poorer countries, meanwhile, is abysmally low – 2.1 percent in Yemen, 4.6 in South Sudan, 5 in Cameroon, 9.9 in Burkina Faso, 10 in Nigeria, 12 in Somalia, 12 in Congo, 13 in Afghanistan and 21 in Ethiopia.

In late 2019, scientists Peter Hotez and Maria Elena Bottazzi set out to create a low-cost vaccine that would help inoculate populations in low- and middle-income countries. In February, they were nominated for a Nobel Peace Prize.
Texas Children's Hospital
The COVID-19 vaccination coverage is particularly low in African countries, and according to Shabir Madhi, a vaccinologist at the University of the Witwatersrand, Johannesburg and co-director of African Local Initiative for Vaccinology Expertise, vaccine access and inequity remains a challenge in Africa. Madhi adds that a lack of vaccine access has affected the pandemic’s trajectory on the continent, but a majority of its people have now developed immunity through natural infection. “This has come at a high cost of loss of lives,” he says.
COVID-19 vaccines mean a significant financial burden for poorer countries, which spend an average of $41 per capita annually on health, while the average cost of every COVID-19 vaccine dose ranges between $2 and $40 in addition to a distribution cost of $3.70 per person for two doses. In December last year, the World Health Organisation (WHO) set a goal of immunizing 70 percent of the population of all countries by mid-2022. This, however, means that low-income countries would have to increase their health expenditure by an average of 56.6 percent to cover the cost, as opposed to 0.8 per cent in high-income countries.
Reflecting on the factors that have driven global inequity in COVID-19 vaccine distribution, Andrea Taylor, assistant director of programs at the Duke Global Health Innovation Center, says that wealthy nations took the risk of investing heavily in the development and scaling up of COVID-19 vaccines – at a time when there was little evidence to show that vaccines would work. This reserved a place for these nations at the front of the queue when doses started rolling off production lines. Lower-income countries, meanwhile, could not afford such investments.
“Now, however, global supply is not the issue,” says Taylor. “We are making plenty of doses to meet global need. The main problem is infrastructure to get the vaccine where it is most needed in a predictable and timely way and to ensure that countries have all the support they need to store, transport, and use the vaccine once it is received.”

Taufique Joarder, vice-chairperson of Bangladesh's Public Health Foundation, sees the need for more trials and data before Corbevax is made available to the general population.
In addition to global inequities in vaccination coverage, there are inequities within nations. Taufique Joarder, vice-chairperson of Bangladesh’s Public Health Foundation, points to the situation in his country, where vaccination coverage in rural and economically disadvantaged communities has suffered owing to weak vaccine-promotion initiatives and the difficulty many people face in registering online for jabs.
Joarder also cites the example of the COVID-19 immunization drive for children aged 12 years and above. “[Children] are given the Pfizer vaccine, which requires an ultralow temperature for storage. This is almost impossible to administer in many parts of the country, especially the rural areas. So, a large proportion of the children are being left out of vaccination,” says Joarder, adding that Corbevax, which is cheaper and requires regular temperature refrigeration “can be an excellent alternative to Pfizer for vaccinating rural children.”
Corbevax vs. mRNA Vaccines
As opposed to most other COVID-19 vaccines, which use the new Messenger RNA (mRNA) vaccine technology, Corbevax is an “old school” vaccine, says Hotez. The vaccine is made through microbial fermentation in yeast, similar to the process used to produce the recombinant hepatitis B vaccine, which has been administered to children in several countries for decades. Hence, says Hotez, the technology to produce Corbevax at large scales is already in place in countries like Vietnam, Bangladesh, India, Indonesia, Brazil, Argentina, among many others.
“So if you want to rapidly develop and produce and empower low- and middle-income countries, this is the technology to do it,” he says.
“Global access to high-quality vaccines will require serious investment in other types of COVID-19 vaccines," says Andrea Taylor.
The COVID-19 vaccines created by Pfizer-BioNTech and Moderna marked the first time that mRNA vaccine technology was approved for use. However, scientists like Young feel that there is “a need to be pragmatic and not seduced by new technologies when older, tried and tested approaches can also be effective.” Taylor, meanwhile, says that although mRNA vaccines have dominated the COVID-19 vaccine market in the U.S., “there is no clear grounding for this preference in the data we have so far.” She adds that there is also growing evidence that the immunity from these shots may not hold up as well over time as that of vaccines using different platforms.
“The mRNA vaccines are well suited to wealthy countries with sufficient ultra-cold storage and transportation infrastructure, but these vaccines are divas and do not travel well in the rest of the world,” says Taylor. “Global access to high-quality vaccines will require serious investment in other types of COVID-19 vaccines, such as the protein subunit platform used by Novavax and Corbevax. These require only standard refrigeration, can be manufactured using existing facilities all over the world, and are easy to transport.”
Joarder adds that Corbevax is cheaper due to the developers’ waived intellectual rights. It could also be used as a booster vaccine in Bangladesh, where only five per cent of the population has currently received booster doses. “If this vaccine is proved effective for heterologous boosting, [meaning] it works well and is well tolerated as a booster with other vaccines that are available in Bangladesh, this can be useful,” says Joarder.
According to Hotez, Corbevax can play several important roles - as a standalone adult or paediatric vaccine, and as a booster for other vaccines. Studies are underway to determine Corbevax’s effectiveness in these regards, he says.
Need for More Data
Biological E conducted two clinical trials involving 3000 subjects in India, and found Corbevax to be “safe and immunogenic,” with 90 percent effectiveness in preventing symptomatic infections from the original strain of COVID-19 and over 80 percent effectiveness against the Delta variant. The vaccine is currently in use in India, and according to Hotez, it’s in the pipeline at different stages in Indonesia, Bangladesh and Botswana.
However, Corbevax is yet to receive emergency use approval from the WHO. Experts such as Joarder see the need for more trials and data before it is made available to the general population. He says that while the WHO’s emergency approval is essential for global scale-up of the vaccine, we need data to determine age-stratified efficacy of the vaccine and whether it can be used for heterologous boosting with other vaccines. “According to the most recent data, the 100 percent circulating variant in Bangladesh is Omicron. We need to know how effective is Corbevax against the Omicron variant,” says Joarder.

Shabir Madhi, a vaccinologist at the University of the Witwatersrand, Johannesburg and co-director of the African Local Initiative for Vaccinology Expertise, says that a majority of people in Africa have now developed immunity through natural infection. “This has come at a high cost of loss of lives."
Shivan Parusnath
Others, meanwhile, believe that availing vaccines to poorer countries is not enough to resolve the inequity. Young, the Warwick virologist, says that the global vaccination rollout has also suffered from a degree of vaccine hesitancy, echoing similar observations by President Biden and Pfizer’s CEO. The problem can be blamed on poor communication about the benefits of vaccination. “The Corbevax vaccine [helps with the issues of] patent protection, vaccine storage and distribution, but governments need to ensure that their people are clearly informed.” Notably, however, some research has found higher vaccine willingness in lower-income countries than in the U.S.
Young also emphasized the importance of establishing local vaccination stations to improve access. For some countries, meanwhile, it may be too late. Speaking about the African continent, Madhi says that Corbevax has arrived following the peak of the crisis and won’t reverse the suffering and death that has transpired because of vaccine hoarding by high-income countries.
“The same goes for all the sudden donations from countries such as France - pretty much of little to no value when the pandemic is at its tail end,” says Madhi. “This, unfortunately, is a repeat of the swine flu pandemic in 2009, when vaccines only became available to Africa after the pandemic had very much subsided.”
When Willem Johan Kolff invented dialysis, the "father" of artificial organs was just getting started.
One of the Netherlands’ most famous pieces of pop culture is “Soldier of Orange.” It’s the title of the country’s most celebrated war memoir, movie and epic stage musical, all of which detail the exploits of the nation’s resistance fighters during World War II.
Willem Johan Kolff was a member of the Dutch resistance, but he doesn’t rate a mention in the “Solider of Orange” canon. Yet his wartime toils in a rural backwater not only changed medicine, but the world.
Kolff had been a physician less than two years before Germany invaded the Netherlands in May 1940. He had been engaged in post-graduate studies at the University of Gronigen but withdrew because he refused to accommodate the demands of the Nazi occupiers. Kolff’s Jewish supervisor made an even starker choice: He committed suicide.
After his departure from the university, Kolff took a job managing a small hospital in Kampen. Located 50 miles from the heavily populated coastal region, the facility was far enough away from the prying eyes of Germans that not only could Kolff care for patients, he could hide fellow resistance fighters and even Jewish refugees in relative safety. Kolff coached many of them to feign convincing terminal illnesses so the Nazis would allow them to remain in the hospital.
Despite the demands of practicing medicine and resistance work, Kolff still found time to conduct research. He had been haunted and inspired when, not long before the Nazi invasion, one of his patients died in agony from kidney disease. Kolff wanted to find a way to save future patients.
He broke his problem down to a simple task: If he could remove 20 grams of urea from a patient’s blood in 24 hours, they would survive. He began experimenting with ways to filter blood and return it to a patient’s body. Since the war had ground all non-military manufacturing to a halt, he was mostly forced to make do with material he could find at the hospital and around Kampen. Kolff eventually built a device from a washing machine parts, juice cans, sausage casings, a valve from an old Ford automobile radiator, and even scrap from a downed German aircraft.
The world’s first dialysis machine was hardly imposing; it resembled a rotating drum for a bingo game or raffle. Yet it carried on the highly sophisticated task of moving a patient’s blood through a semi-permeable membrane (about a 50-foot length of sausage casings) into a saline solution that drew out urea while leaving the blood cells untouched.
In emigrating to the U.S. to practice medicine, Kolff's intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
Kolff began using the machine to treat patients in 1943, most of whom had lapsed into comas due to their kidney failure. But like most groundbreaking medical devices, it was not an immediate success. By the end of the war, Kolff had dialyzed more than a dozen patients, but all had died. He briefly suspended use of the device after the Allied invasion of Europe, but he continued to refine its operation and the administration of blood thinners to patients.
In September 1945, Kolff dialyzed another comatose patient, 67-year-old Sofia Maria Schafstadt. She regained consciousness after 11 hours, and would live well into the 1950s with Kolff’s assistance. Yet this triumph contained a dark irony: At the time of her treatment, Schafstadt had been imprisoned for collaborating with the Germans.
With a tattered Europe struggling to overcome the destruction of the war, Kolff and his family emigrated to the U.S. in 1950, where he began working for the Cleveland Clinic while undergoing the naturalization process so he could practice medicine in the U.S. His intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
By the mid-1950s, dialysis machines had become reliable and life-saving medical devices, and Kolff had become a U.S. citizen. About that time he invented a membrane oxygenator that could be used in heart bypass surgeries. This was a critical component of the heart-lung machine, which would make heart transplants possible and bypass surgeries routine. He also invented among the very first practical artificial hearts, which in 1957 kept a dog alive for 90 minutes.
Kolff moved to the University of Utah in 1967 to become director of its Institute for Biomedical Engineering. It was a promising time for such a move, as the first successful transplant of a donor heart to a human occurred that year. But he was interested in going a step further and creating an artificial heart for human use.
It took more than a decade of tinkering and research, but in 1982, a team of physicians and engineers led by Kolff succeeded in implanting the first artificial heart in dentist Barney Clark, whose failing health disqualified him from a heart transplant. Although Clark died in March 1983 after 112 days tethered to the device, that it kept him alive generated international headlines. While graduate student Robert Jarvik received the named credit for the heart, he was directly supervised by Kolff, whose various endeavors into artificial organ research at the University of Utah were segmented into numerous teams.
Forty years later, several artificial hearts have been approved for use by the Food and Drug Administration, although all are a “bridge” that allow patients to wait for a transplant.
Kolff continued researching and tinkering with biomedical devices – including artificial eyes and ears – until he retired in 1997 at the age of 86. When he died in 2009, the medical community acknowledged that he was not only a pioneer in biotechnology, but the “father” of artificial organs.