New drug for schizophrenia could meet desperate need for better treatments

The field of treating schizophrenia with drugs has been stuck in a long drought but, this month, a late-stage clinical trial found a new drug called KarXT could treat a range of symptoms.
Schizophrenia is a debilitating mental health condition that affects around 24 million people worldwide. Patients experience hallucinations and delusions when they develop schizophrenia, with experts referring to these new thoughts and behaviors as positive symptoms. They also suffer from negative symptoms in which they lose important functions, suffering from dulled emotions, lack of purpose and social withdrawal.
Currently available drugs can control only a portion of these symptoms but, on August 8th, Karuna Therapeutics announced its completion of a phase 3 clinical trial that found a new drug called KarXT could treat both positive and negative symptoms of schizophrenia. It could mean substantial progress against a problem that has stymied scientists for decades.
A long-standing problem
Since the 1950s, antipsychotics have been used to treat schizophrenia. People who suffer from it are thought to have too much of a brain chemical called dopamine, and antipsychotics work by blocking dopamine receptors in the brain. They can be effective in treating positive symptoms but have little impact on the negative ones, which can be devastating for a patient’s quality of life, making it difficult to maintain employment and have successful relationships. About 30 percent of schizophrenia patients don't actually respond to antipsychotics at all. Current drugs can also have adverse side effects including elevated cholesterol, high blood pressure, diabetes and movements that patients cannot control.
The recent clinical trial heralds a new treatment approach. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” says Andrew Miller, COO of Karuna.
Scientists have been looking to develop alternatives. However, “the field of drug treatment of schizophrenia is currently in the doldrums,” says Peter McKenna, a senior researcher at FIDMAG Research Foundation in Spain which specialises in mental health.
In the 2000s there was a major push to target a brain receptor for a chemical called glutamate. Evidence suggested that this receptor is abnormal in the brains of schizophrenia patients, but attempts to try glutamate failed in clinical trials.
After that, many pharmaceutical companies dropped out of the race for a more useful treatment. But some companies continued to search, such as Karuna Therapeutics, led by founder and Chief Operating Officer Andrew Miller and CEO Steve Paul. The recent clinical trial suggests their persistence has led to an important breakthrough with their drug, KarXT. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” Miller says.
How it works
Neurotransmitters are chemical messengers that pass signals between neurons. To work effectively, neurotransmitters need a receptor to bind to. A neurotransmitter called acetylcholine seems to be especially important in schizophrenia. It interacts with sites called muscarinic receptors, which are involved in the network of nerves that calm your body after a stressful event. Post mortem studies in people with schizophrenia have shown that two muscarinic receptors in the brain, the M1 and M4 receptors, are activated at unusually low levels because they don’t receive enough signals from acetylcholine.
The M4 receptor appears to play a role in psychosis. The M1 receptor is also associated with psychosis but is primarily thought to be involved in cognition. KarXT, taken orally, works by activating both of these receptors to signal properly. It is this twofold action that seems to explain its effectiveness. “[The drug’s] design enables the preferential stimulation of these muscarinic receptors in the brain,” Miller says.
How it developed
It all started in the early 1990s when Paul was at pharmaceutical company Eli Lilly. He discovered that Xanomeline, the drug they were testing on Alzheimer's patients, had antipsychotic effects. It worked by stimulating M1 and M4 receptors, so he and his colleagues decided to test Xanomeline on schizophrenia patients, supported by research on the connection between muscarinic receptors and psychosis. They found that Xanomeline reduced both positive and negative symptoms.
Unfortunately, it also caused significant side effects. The problem was that stimulating the M1 and M4 receptors in the brain also stimulated muscarinic receptors in the body that led to severe vomiting, diarrhea and even the temporary loss of consciousness.
In the end, Eli Lilly discontinued the clinical trials for the drug, but Miller set up Karuna Therapeutics to develop a solution. “I was determined to find a way to harness the therapeutic benefit demonstrated in studies of Xanomeline, while eliminating side effects that limited its development,” Miller says.
He analysed over 7,000 possible ways of mixing Xanomeline with other agents before settling on KarXT. It combines Xanomeline with a drug called Trospium Chloride, which blocks muscarinic receptors in the body – taking care of the side effects such as vomiting – but leaves them unblocked in the brain. Paul was so excited by Miller’s progress that he joined Karuna after leaving Eli Lilly and founding two previous startups.
“It's a very important approach,” says Rick Adams, Future Leaders Fellow in the Institute of Cognitive Neuroscience and Centre for Medical Image Computing at University College London. “We are in desperate need of alternative drug targets and this target is one of the best. There are other alternative targets, but not many are as close to being successful as the muscarinic receptor drug.”
Clinical Trial
Following a successful phase 2 clinical trial in 2019, the most recent trial involved 126 patients who were given KarXT, and 126 who were given a placebo. Compared to the placebo, patients taking KarXT had a significant 9.6 point reduction in the positive and negative syndrome scale (PANSS), the standard for rating schizophrenic symptoms.
KarXT also led to statistically significant declines in positive and negative symptoms compared to the placebo. “The results suggest that KarXT could be a potentially game-changing option in the management of both positive and negative symptoms of schizophrenia,” Miller says.
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, is optimistic about the side effects but highlights the need for more safety trials.
McKenna, the researcher at FIDMAG Foundation, agrees about the drug’s potential. “The new [phase 3] study is positive,” he says. “It is reassuring that one is not dealing with a drug that works in one trial and then inexplicably fails in the next one.”
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, said the drug is an unprecedented step forward. “KarXT is one of the first drugs with a novel mechanism of action to show promise in clinical trials.”
Even though the drug blocks muscarine receptors in the body, some patients still suffered from adverse side effects like vomiting, dizziness and diarrhea. But in general, these effects were mild to moderate, especially compared to dopamine-blocking antipsychotics or Xanomeline on its own.
McCutcheon is optimistic about the side effects but highlights the need for more safety trials. “The trial results suggest that gastrointestinal side effects appear to be manageable,” he says. “We know, however, from previous antipsychotic drugs that the full picture regarding the extent of side effects can sometimes take longer to become apparent to clinicians and patients. Careful ongoing assessment during a longer period of treatment will therefore be important.”
The Future
The team is currently conducting three other trials to evaluate the efficacy and long-term safety of KarXT. Their goal is to receive FDA approval next year.
Karuna is also conducting trials to evaluate the effectiveness of KarXT in treating psychosis in patients suffering from Alzheimer’s.
The big hope is that they will soon be able to provide a radically different drug to help many patients with schizophrenia. “We are another step closer to potentially providing the first new class of medicine in more than 50 years to the millions of people worldwide living with schizophrenia,” says Miller.
Biohackers Made a Cheap and Effective Home Covid Test -- But No One Is Allowed to Use It
A stock image of a home test for COVID-19.
Last summer, when fast and cheap Covid tests were in high demand and governments were struggling to manufacture and distribute them, a group of independent scientists working together had a bit of a breakthrough.
Working on the Just One Giant Lab platform, an online community that serves as a kind of clearing house for open science researchers to find each other and work together, they managed to create a simple, one-hour Covid test that anyone could take at home with just a cup of hot water. The group tested it across a network of home and professional laboratories before being listed as a semi-finalist team for the XPrize, a competition that rewards innovative solutions-based projects. Then, the group hit a wall: they couldn't commercialize the test.
They wanted to keep their project open source, making it accessible to people around the world, so they decided to forgo traditional means of intellectual property protection and didn't seek patents. (They couldn't afford lawyers anyway). And, as a loose-knit group that was not supported by a traditional scientific institution, working in community labs and homes around the world, they had no access to resources or financial support for manufacturing or distributing their test at scale.
But without ethical and regulatory approval for clinical testing, manufacture, and distribution, they were legally unable to create field tests for real people, leaving their inexpensive, $16-per-test, innovative product languishing behind, while other, more expensive over-the-counter tests made their way onto the market.
Who Are These Radical Scientists?
Independent, decentralized biomedical research has come of age. Also sometimes called DIYbio, biohacking, or community biology, depending on whom you ask, open research is today a global movement with thousands of members, from scientists with advanced degrees to middle-grade students. Their motivations and interests vary across a wide spectrum, but transparency and accessibility are key to the ethos of the movement. Teams are agile, focused on shoestring-budget R&D, and aim to disrupt business as usual in the ivory towers of the scientific establishment.
Ethics oversight is critical to ensuring that research is conducted responsibly, even by biohackers.
Initiatives developed within the community, such as Open Insulin, which hopes to engineer processes for affordable, small-batch insulin production, "Slybera," a provocative attempt to reverse engineer a $1 million dollar gene therapy, and the hundreds of projects posted on the collaboration platform Just One Giant Lab during the pandemic, all have one thing in common: to pursue testing in humans, they need an ethics oversight mechanism.
These groups, most of which operate collaboratively in community labs, homes, and online, recognize that some sort of oversight or guidance is useful—and that it's the right thing to do.
But also, and perhaps more immediately, they need it because federal rules require ethics oversight of any biomedical research that's headed in the direction of the consumer market. In addition, some individuals engaged in this work do want to publish their research in traditional scientific journals, which—you guessed it—also require that research has undergone an ethics evaluation. Ethics oversight is critical to ensuring that research is conducted responsibly, even by biohackers.
Bridging the Ethics Gap
The problem is that traditional oversight mechanisms, such as institutional review boards at government or academic research institutions, as well as the private boards utilized by pharmaceutical companies, are not accessible to most independent researchers. Traditional review boards are either closed to the public, or charge fees that are out of reach for many citizen science initiatives. This has created an "ethics gap" in nontraditional scientific research.
Biohackers are seen in some ways as the direct descendents of "white hat" computer hackers, or those focused on calling out security holes and contributing solutions to technical problems within self-regulating communities. In the case of health and biotechnology, those problems include both the absence of treatments and the availability of only expensive treatments for certain conditions. As the DIYbio community grows, there needs to be a way to provide assurance that, when the work is successful, the public is able to benefit from it eventually. The team that developed the one-hour Covid test found a potential commercial partner and so might well overcome the oversight hurdle, but it's been 14 months since they developed the test--and counting.
In short, without some kind of oversight mechanism for the work of independent biomedical researchers, the solutions they innovate will never have the opportunity to reach consumers.
In a new paper in the journal Citizen Science: Theory & Practice, we consider the issue of the ethics gap and ask whether ethics oversight is something nontraditional researchers want, and if so, what forms it might take. Given that individuals within these communities sometimes vehemently disagree with each other, is consensus on these questions even possible?
We learned that there is no "one size fits all" solution for ethics oversight of nontraditional research. Rather, the appropriateness of any oversight model will depend on each initiative's objectives, needs, risks, and constraints.
We also learned that nontraditional researchers are generally willing (and in some cases eager) to engage with traditional scientific, legal, and bioethics experts on ethics, safety, and related questions.
We suggest that these experts make themselves available to help nontraditional researchers build infrastructure for ethics self-governance and identify when it might be necessary to seek outside assistance.
Independent biomedical research has promise, but like any emerging science, it poses novel ethical questions and challenges. Existing research ethics and oversight frameworks may not be well-suited to answer them in every context, so we need to think outside the box about what we can create for the future. That process should begin by talking to independent biomedical researchers about their activities, priorities, and concerns with an eye to understanding how best to support them.
Christi Guerrini, JD, MPH studies biomedical citizen science and is an Associate Professor at Baylor College of Medicine. Alex Pearlman, MA, is a science journalist and bioethicist who writes about emerging issues in biotechnology. They have recently launched outlawbio.org, a place for discussion about nontraditional research.
Sept. 13th Event: Delta, Vaccines, and Breakthrough Infections
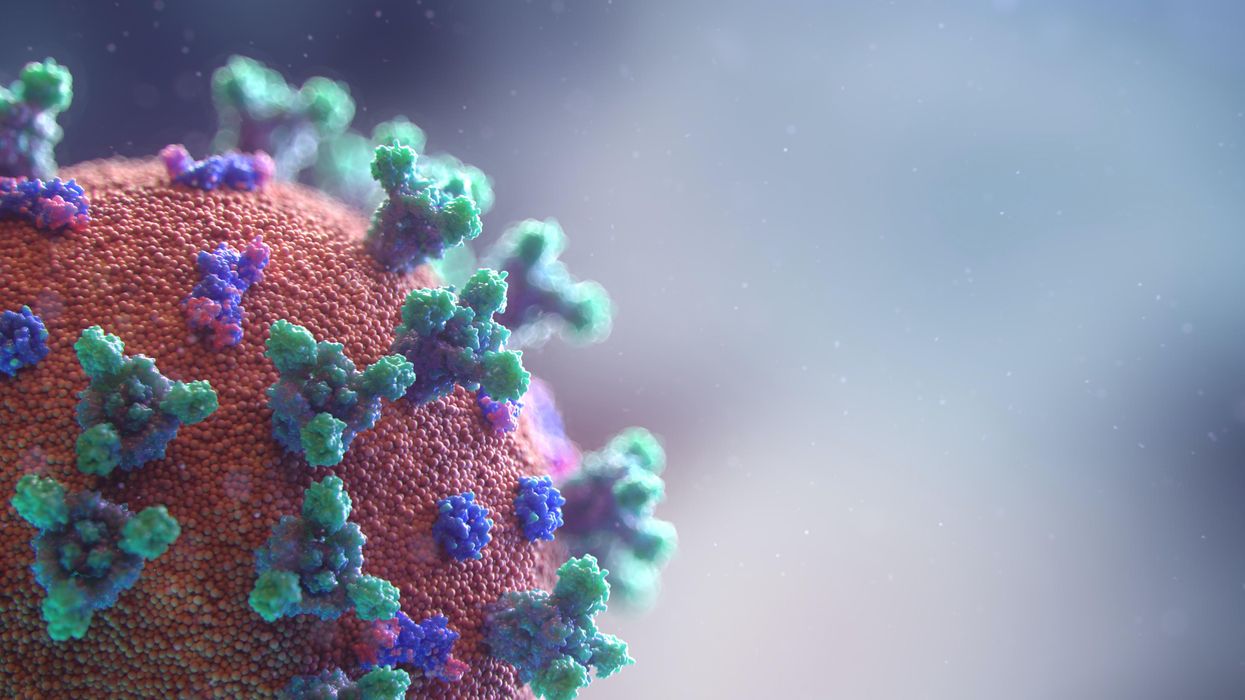
A visualization of the virus that causes COVID-19.
This virtual event will convene leading scientific and medical experts to address the public's questions and concerns about COVID-19 vaccines, Delta, and breakthrough infections. Audience Q&A will follow the panel discussion. Your questions can be submitted in advance at the registration link.
DATE:
Monday, September 13th, 2021
12:30 p.m. - 1:45 p.m. EDT
REGISTER:

Dr. Amesh Adalja, M.D., FIDSA, Senior Scholar, Johns Hopkins Center for Health Security; Adjunct Assistant Professor, Johns Hopkins Bloomberg School of Public Health; Affiliate of the Johns Hopkins Center for Global Health. His work is focused on emerging infectious disease, pandemic preparedness, and biosecurity.

Dr. Nahid Bhadelia, M.D., MALD, Founding Director, Boston University Center for Emerging Infectious Diseases Policy and Research (CEID); Associate Director, National Emerging Infectious Diseases Laboratories (NEIDL), Boston University; Associate Professor, Section of Infectious Diseases, Boston University School of Medicine. She is an internationally recognized leader in highly communicable and emerging infectious diseases (EIDs) with clinical, field, academic, and policy experience in pandemic preparedness.

Dr. Akiko Iwasaki, Ph.D., Waldemar Von Zedtwitz Professor of Immunobiology and Molecular, Cellular and Developmental Biology and Professor of Epidemiology (Microbial Diseases), Yale School of Medicine; Investigator, Howard Hughes Medical Institute. Her laboratory researches how innate recognition of viral infections lead to the generation of adaptive immunity, and how adaptive immunity mediates protection against subsequent viral challenge.
 Dr. Marion Pepper, Ph.D., Associate Professor, Department of Immunology, University of Washington. Her lab studies how cells of the adaptive immune system, called CD4+ T cells and B cells, form immunological memory by visualizing their differentiation, retention, and function.
Dr. Marion Pepper, Ph.D., Associate Professor, Department of Immunology, University of Washington. Her lab studies how cells of the adaptive immune system, called CD4+ T cells and B cells, form immunological memory by visualizing their differentiation, retention, and function.
This event is the third of a four-part series co-hosted by Leaps.org, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.