New drug for schizophrenia could meet desperate need for better treatments

The field of treating schizophrenia with drugs has been stuck in a long drought but, this month, a late-stage clinical trial found a new drug called KarXT could treat a range of symptoms.
Schizophrenia is a debilitating mental health condition that affects around 24 million people worldwide. Patients experience hallucinations and delusions when they develop schizophrenia, with experts referring to these new thoughts and behaviors as positive symptoms. They also suffer from negative symptoms in which they lose important functions, suffering from dulled emotions, lack of purpose and social withdrawal.
Currently available drugs can control only a portion of these symptoms but, on August 8th, Karuna Therapeutics announced its completion of a phase 3 clinical trial that found a new drug called KarXT could treat both positive and negative symptoms of schizophrenia. It could mean substantial progress against a problem that has stymied scientists for decades.
A long-standing problem
Since the 1950s, antipsychotics have been used to treat schizophrenia. People who suffer from it are thought to have too much of a brain chemical called dopamine, and antipsychotics work by blocking dopamine receptors in the brain. They can be effective in treating positive symptoms but have little impact on the negative ones, which can be devastating for a patient’s quality of life, making it difficult to maintain employment and have successful relationships. About 30 percent of schizophrenia patients don't actually respond to antipsychotics at all. Current drugs can also have adverse side effects including elevated cholesterol, high blood pressure, diabetes and movements that patients cannot control.
The recent clinical trial heralds a new treatment approach. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” says Andrew Miller, COO of Karuna.
Scientists have been looking to develop alternatives. However, “the field of drug treatment of schizophrenia is currently in the doldrums,” says Peter McKenna, a senior researcher at FIDMAG Research Foundation in Spain which specialises in mental health.
In the 2000s there was a major push to target a brain receptor for a chemical called glutamate. Evidence suggested that this receptor is abnormal in the brains of schizophrenia patients, but attempts to try glutamate failed in clinical trials.
After that, many pharmaceutical companies dropped out of the race for a more useful treatment. But some companies continued to search, such as Karuna Therapeutics, led by founder and Chief Operating Officer Andrew Miller and CEO Steve Paul. The recent clinical trial suggests their persistence has led to an important breakthrough with their drug, KarXT. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” Miller says.
How it works
Neurotransmitters are chemical messengers that pass signals between neurons. To work effectively, neurotransmitters need a receptor to bind to. A neurotransmitter called acetylcholine seems to be especially important in schizophrenia. It interacts with sites called muscarinic receptors, which are involved in the network of nerves that calm your body after a stressful event. Post mortem studies in people with schizophrenia have shown that two muscarinic receptors in the brain, the M1 and M4 receptors, are activated at unusually low levels because they don’t receive enough signals from acetylcholine.
The M4 receptor appears to play a role in psychosis. The M1 receptor is also associated with psychosis but is primarily thought to be involved in cognition. KarXT, taken orally, works by activating both of these receptors to signal properly. It is this twofold action that seems to explain its effectiveness. “[The drug’s] design enables the preferential stimulation of these muscarinic receptors in the brain,” Miller says.
How it developed
It all started in the early 1990s when Paul was at pharmaceutical company Eli Lilly. He discovered that Xanomeline, the drug they were testing on Alzheimer's patients, had antipsychotic effects. It worked by stimulating M1 and M4 receptors, so he and his colleagues decided to test Xanomeline on schizophrenia patients, supported by research on the connection between muscarinic receptors and psychosis. They found that Xanomeline reduced both positive and negative symptoms.
Unfortunately, it also caused significant side effects. The problem was that stimulating the M1 and M4 receptors in the brain also stimulated muscarinic receptors in the body that led to severe vomiting, diarrhea and even the temporary loss of consciousness.
In the end, Eli Lilly discontinued the clinical trials for the drug, but Miller set up Karuna Therapeutics to develop a solution. “I was determined to find a way to harness the therapeutic benefit demonstrated in studies of Xanomeline, while eliminating side effects that limited its development,” Miller says.
He analysed over 7,000 possible ways of mixing Xanomeline with other agents before settling on KarXT. It combines Xanomeline with a drug called Trospium Chloride, which blocks muscarinic receptors in the body – taking care of the side effects such as vomiting – but leaves them unblocked in the brain. Paul was so excited by Miller’s progress that he joined Karuna after leaving Eli Lilly and founding two previous startups.
“It's a very important approach,” says Rick Adams, Future Leaders Fellow in the Institute of Cognitive Neuroscience and Centre for Medical Image Computing at University College London. “We are in desperate need of alternative drug targets and this target is one of the best. There are other alternative targets, but not many are as close to being successful as the muscarinic receptor drug.”
Clinical Trial
Following a successful phase 2 clinical trial in 2019, the most recent trial involved 126 patients who were given KarXT, and 126 who were given a placebo. Compared to the placebo, patients taking KarXT had a significant 9.6 point reduction in the positive and negative syndrome scale (PANSS), the standard for rating schizophrenic symptoms.
KarXT also led to statistically significant declines in positive and negative symptoms compared to the placebo. “The results suggest that KarXT could be a potentially game-changing option in the management of both positive and negative symptoms of schizophrenia,” Miller says.
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, is optimistic about the side effects but highlights the need for more safety trials.
McKenna, the researcher at FIDMAG Foundation, agrees about the drug’s potential. “The new [phase 3] study is positive,” he says. “It is reassuring that one is not dealing with a drug that works in one trial and then inexplicably fails in the next one.”
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, said the drug is an unprecedented step forward. “KarXT is one of the first drugs with a novel mechanism of action to show promise in clinical trials.”
Even though the drug blocks muscarine receptors in the body, some patients still suffered from adverse side effects like vomiting, dizziness and diarrhea. But in general, these effects were mild to moderate, especially compared to dopamine-blocking antipsychotics or Xanomeline on its own.
McCutcheon is optimistic about the side effects but highlights the need for more safety trials. “The trial results suggest that gastrointestinal side effects appear to be manageable,” he says. “We know, however, from previous antipsychotic drugs that the full picture regarding the extent of side effects can sometimes take longer to become apparent to clinicians and patients. Careful ongoing assessment during a longer period of treatment will therefore be important.”
The Future
The team is currently conducting three other trials to evaluate the efficacy and long-term safety of KarXT. Their goal is to receive FDA approval next year.
Karuna is also conducting trials to evaluate the effectiveness of KarXT in treating psychosis in patients suffering from Alzheimer’s.
The big hope is that they will soon be able to provide a radically different drug to help many patients with schizophrenia. “We are another step closer to potentially providing the first new class of medicine in more than 50 years to the millions of people worldwide living with schizophrenia,” says Miller.
Vaccines Without Vaccinations Won’t End the Pandemic
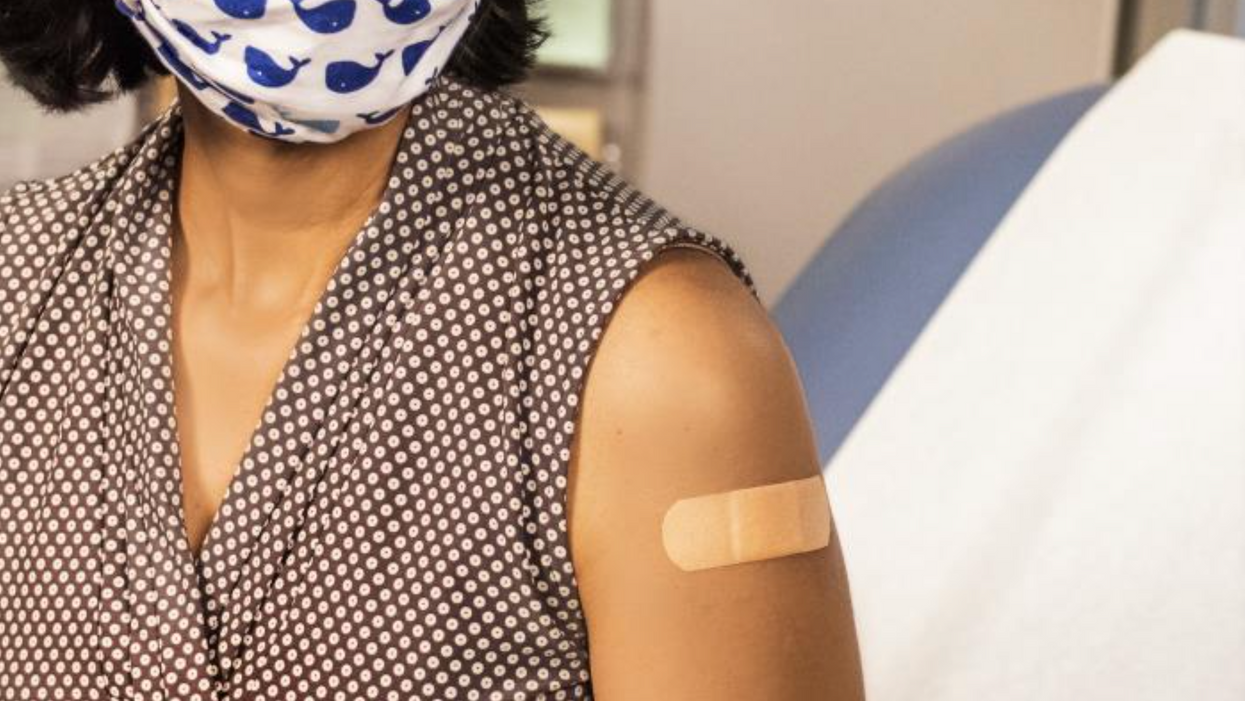
In this 2020 photograph, a bandage is placed on a patient who has just received a vaccine.
COVID-19 vaccine development has advanced at a record-setting pace, thanks to our nation's longstanding support for basic vaccine science coupled with massive public and private sector investments.
Yet, policymakers aren't according anywhere near the same level of priority to investments in the social, behavioral, and data science needed to better understand who and what influences vaccination decision-making. "If we want to be sure vaccines become vaccinations, this is exactly the kind of work that's urgently needed," says Dr. Bruce Gellin, President of Global Immunization at the Sabin Vaccine Institute.
Simply put: it's possible vaccines will remain in refrigerators and not be delivered to the arms of rolled-up sleeves if we don't quickly ramp up vaccine confidence research and broadly disseminate the findings.
According to the most recent Gallup poll, the share of U.S. adults who say they would get a COVID-19 vaccine rose to 58 percent this month from 50 percent in September, with non-white Americans and those ages 45-65 even less willing to be vaccinated. While there is still much we don't understand about COVID-19, we do know that without high levels of immunity in the population, a return to some semblance of normalcy is wishful thinking.
Research from prior vaccination campaigns such as H1N1, HPV, and the annual flu points us in the right direction. Key components of successful vaccination efforts require 1) Identifying the concerns of particular segments of the population; 2) Tailoring messages and incentives to address those concerns, and 3) Reaching out through trusted sources – health care providers, public health departments, and others in the community.
Research during the H1N1 flu found preparing people for some uncertainty actually improved trust, according to Dr. Sandra Crouse Quinn, professor and chair, Family Science, University of Maryland. Dr. Crouse Quinn's research during that period also underscored the need to address the specific vaccine concerns of racial and ethnic groups.
The stunning scientific achievement of COVID-19 vaccines anticipated to be ready in record time needs to be backed up by an equally ambitious and evidence-based effort to build the public's confidence in the vaccines.
Data science has provided crucial insight about the social media universe. Dr. Neil Johnson, a scientist at George Washington University, found that despite having fewer followers, anti-vaccination pages are more numerous and growing faster than pro-vaccination pages. They are more often linked to in discussions on other Facebook pages – such as school parent associations – where people are undecided about vaccination.
We've learned about building vaccine confidence from earlier campaigns. Now, however, we are faced with a unique and challenging set of obstacles to unpack quickly: How do we communicate the importance of eventual COVID-19 vaccines to Americans in light of the muddled-to-poor messaging from political leaders, the weaponizing of relatively simple public health recommendations, the enormous disproportionate toll on people of color, and the torrent of online misinformation? We urgently need data reflective of today's circumstances along with the policy to ensure it is quickly and effectively disseminated to the public health and clinical workforce.
Last year prompted in part by the measles outbreaks, Reps. Michael C. Burgess (R-TX) and Kim Shrier (D-WA), both physicians, introduced the bipartisan Vaccines Act to develop a national surveillance system to monitor vaccination rates and conduct a national campaign to increase awareness of the importance of vaccines. Unfortunately, that legislation wasn't passed. In response to COVID-19, Senate HELP Committee Ranking member Patty Murray (D-WA) has sought funds to strengthen vaccine confidence and combat misinformation with federally supported communication, research, and outreach efforts. Leading experts outside of Congress have called for this type of research, including the Sabin-Aspen Vaccine Science Policy Institute. Most recently, the National Academy of Sciences, in its report regarding the equitable distribution of the COVID-19 vaccine, included as one of its recommendations the need for "a rapid-response program to advance the science behind vaccine confidence."
Addressing trust in vaccination has never been as challenging nor as consequential. The stunning scientific achievement of COVID-19 vaccines anticipated to be ready in record time needs to be backed up by an equally ambitious and evidence-based effort to build the public's confidence in the vaccines. In its remaining days, the Trump Administration should invest in building vaccine confidence with current resources, targeting efforts to ensure COVID vaccines reduce rather than exacerbate racial and ethnic health disparities. Congress must also act to provide the additional research and outreach resources needed as well as pass the Vaccines Act so we are better prepared in the future.
If we don't succeed, COVID-19 will continue wreaking havoc on our health, our society, and our economy. We will also permanently jeopardize public trust in vaccines – one of the most successful medical interventions in human history.
New Video: Secret Heroes of the Pandemic
These 6 people deserve to be widely known and celebrated for their brave and ingenious contributions to battling the pandemic.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.