New drug for schizophrenia could meet desperate need for better treatments

The field of treating schizophrenia with drugs has been stuck in a long drought but, this month, a late-stage clinical trial found a new drug called KarXT could treat a range of symptoms.
Schizophrenia is a debilitating mental health condition that affects around 24 million people worldwide. Patients experience hallucinations and delusions when they develop schizophrenia, with experts referring to these new thoughts and behaviors as positive symptoms. They also suffer from negative symptoms in which they lose important functions, suffering from dulled emotions, lack of purpose and social withdrawal.
Currently available drugs can control only a portion of these symptoms but, on August 8th, Karuna Therapeutics announced its completion of a phase 3 clinical trial that found a new drug called KarXT could treat both positive and negative symptoms of schizophrenia. It could mean substantial progress against a problem that has stymied scientists for decades.
A long-standing problem
Since the 1950s, antipsychotics have been used to treat schizophrenia. People who suffer from it are thought to have too much of a brain chemical called dopamine, and antipsychotics work by blocking dopamine receptors in the brain. They can be effective in treating positive symptoms but have little impact on the negative ones, which can be devastating for a patient’s quality of life, making it difficult to maintain employment and have successful relationships. About 30 percent of schizophrenia patients don't actually respond to antipsychotics at all. Current drugs can also have adverse side effects including elevated cholesterol, high blood pressure, diabetes and movements that patients cannot control.
The recent clinical trial heralds a new treatment approach. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” says Andrew Miller, COO of Karuna.
Scientists have been looking to develop alternatives. However, “the field of drug treatment of schizophrenia is currently in the doldrums,” says Peter McKenna, a senior researcher at FIDMAG Research Foundation in Spain which specialises in mental health.
In the 2000s there was a major push to target a brain receptor for a chemical called glutamate. Evidence suggested that this receptor is abnormal in the brains of schizophrenia patients, but attempts to try glutamate failed in clinical trials.
After that, many pharmaceutical companies dropped out of the race for a more useful treatment. But some companies continued to search, such as Karuna Therapeutics, led by founder and Chief Operating Officer Andrew Miller and CEO Steve Paul. The recent clinical trial suggests their persistence has led to an important breakthrough with their drug, KarXT. “We believe it marks an important advancement for patients given its new and completely different mechanism of action from current therapies,” Miller says.
How it works
Neurotransmitters are chemical messengers that pass signals between neurons. To work effectively, neurotransmitters need a receptor to bind to. A neurotransmitter called acetylcholine seems to be especially important in schizophrenia. It interacts with sites called muscarinic receptors, which are involved in the network of nerves that calm your body after a stressful event. Post mortem studies in people with schizophrenia have shown that two muscarinic receptors in the brain, the M1 and M4 receptors, are activated at unusually low levels because they don’t receive enough signals from acetylcholine.
The M4 receptor appears to play a role in psychosis. The M1 receptor is also associated with psychosis but is primarily thought to be involved in cognition. KarXT, taken orally, works by activating both of these receptors to signal properly. It is this twofold action that seems to explain its effectiveness. “[The drug’s] design enables the preferential stimulation of these muscarinic receptors in the brain,” Miller says.
How it developed
It all started in the early 1990s when Paul was at pharmaceutical company Eli Lilly. He discovered that Xanomeline, the drug they were testing on Alzheimer's patients, had antipsychotic effects. It worked by stimulating M1 and M4 receptors, so he and his colleagues decided to test Xanomeline on schizophrenia patients, supported by research on the connection between muscarinic receptors and psychosis. They found that Xanomeline reduced both positive and negative symptoms.
Unfortunately, it also caused significant side effects. The problem was that stimulating the M1 and M4 receptors in the brain also stimulated muscarinic receptors in the body that led to severe vomiting, diarrhea and even the temporary loss of consciousness.
In the end, Eli Lilly discontinued the clinical trials for the drug, but Miller set up Karuna Therapeutics to develop a solution. “I was determined to find a way to harness the therapeutic benefit demonstrated in studies of Xanomeline, while eliminating side effects that limited its development,” Miller says.
He analysed over 7,000 possible ways of mixing Xanomeline with other agents before settling on KarXT. It combines Xanomeline with a drug called Trospium Chloride, which blocks muscarinic receptors in the body – taking care of the side effects such as vomiting – but leaves them unblocked in the brain. Paul was so excited by Miller’s progress that he joined Karuna after leaving Eli Lilly and founding two previous startups.
“It's a very important approach,” says Rick Adams, Future Leaders Fellow in the Institute of Cognitive Neuroscience and Centre for Medical Image Computing at University College London. “We are in desperate need of alternative drug targets and this target is one of the best. There are other alternative targets, but not many are as close to being successful as the muscarinic receptor drug.”
Clinical Trial
Following a successful phase 2 clinical trial in 2019, the most recent trial involved 126 patients who were given KarXT, and 126 who were given a placebo. Compared to the placebo, patients taking KarXT had a significant 9.6 point reduction in the positive and negative syndrome scale (PANSS), the standard for rating schizophrenic symptoms.
KarXT also led to statistically significant declines in positive and negative symptoms compared to the placebo. “The results suggest that KarXT could be a potentially game-changing option in the management of both positive and negative symptoms of schizophrenia,” Miller says.
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, is optimistic about the side effects but highlights the need for more safety trials.
McKenna, the researcher at FIDMAG Foundation, agrees about the drug’s potential. “The new [phase 3] study is positive,” he says. “It is reassuring that one is not dealing with a drug that works in one trial and then inexplicably fails in the next one.”
Robert McCutcheon, a psychiatrist and neuroscientist at Oxford University, said the drug is an unprecedented step forward. “KarXT is one of the first drugs with a novel mechanism of action to show promise in clinical trials.”
Even though the drug blocks muscarine receptors in the body, some patients still suffered from adverse side effects like vomiting, dizziness and diarrhea. But in general, these effects were mild to moderate, especially compared to dopamine-blocking antipsychotics or Xanomeline on its own.
McCutcheon is optimistic about the side effects but highlights the need for more safety trials. “The trial results suggest that gastrointestinal side effects appear to be manageable,” he says. “We know, however, from previous antipsychotic drugs that the full picture regarding the extent of side effects can sometimes take longer to become apparent to clinicians and patients. Careful ongoing assessment during a longer period of treatment will therefore be important.”
The Future
The team is currently conducting three other trials to evaluate the efficacy and long-term safety of KarXT. Their goal is to receive FDA approval next year.
Karuna is also conducting trials to evaluate the effectiveness of KarXT in treating psychosis in patients suffering from Alzheimer’s.
The big hope is that they will soon be able to provide a radically different drug to help many patients with schizophrenia. “We are another step closer to potentially providing the first new class of medicine in more than 50 years to the millions of people worldwide living with schizophrenia,” says Miller.
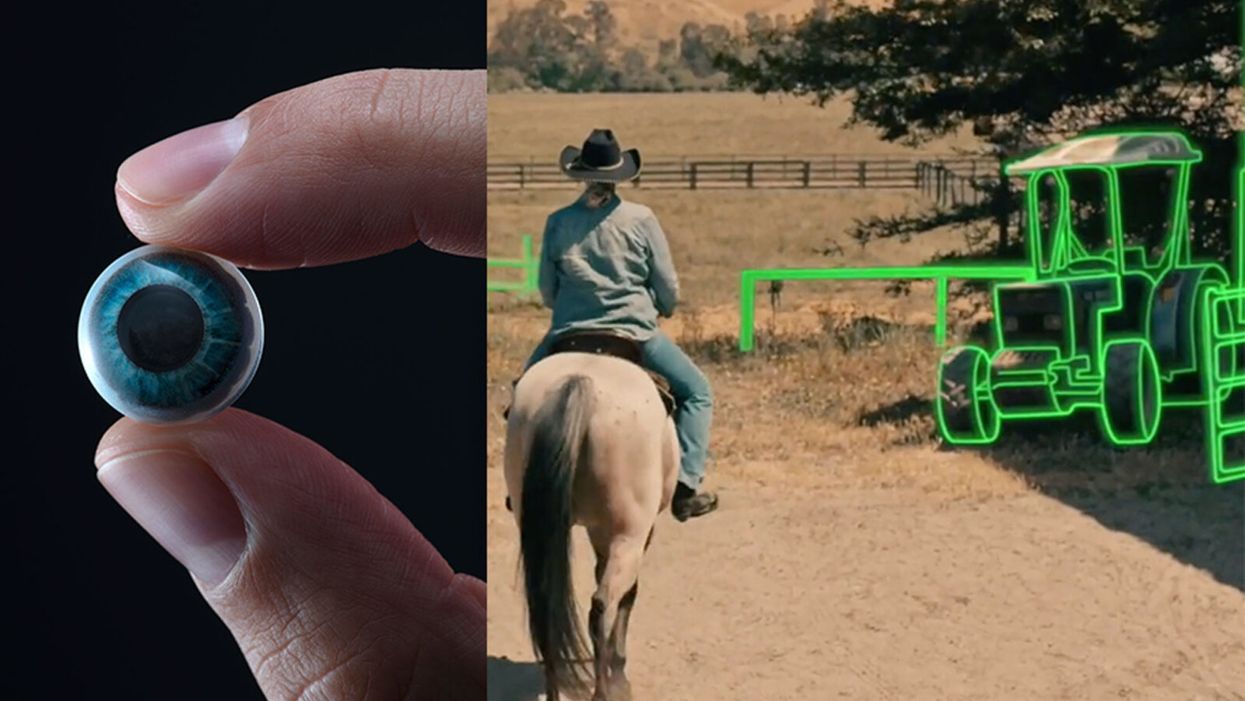
World’s First “Augmented Reality” Contact Lens Aims to Revolutionize Much More Than Medicine
On the left, a picture of the Mojo lens smart contact; and a simulated image of a woman with low vision who is wearing the contact to highlight objects in her field of vision.
Imagine a world without screens. Instead of endlessly staring at your computer or craning your neck down to scroll through social media feeds and emails, information simply appears in front of your eyes when you need it and disappears when you don't.
"The vision is super clear...I was reading the poem with my eyes closed."
No more rude interruptions during dinner, no more bumping into people on the street while trying to follow GPS directions — just the information you want, when you need it, projected directly onto your visual field.
While this screenless future sounds like science fiction, it may soon be a reality thanks to the new Silicon Valley startup Mojo Vision, creator of the world's first smart contact lens. With a 14,000 pixel-per-inch display with eye-tracking, image stabilization, and a custom wireless radio, the Mojo smart lens bills itself the "smallest and densest dynamic display ever made." Unlike current augmented reality wearables such as Google Glass or ThirdEye, which project images onto a glass screen, the Mojo smart lens can project images directly onto the retina.
A current prototype displayed at the Consumer Electronics Show earlier this year in Las Vegas includes a tiny screen positioned right above the most sensitive area of the pupil. "[The Mojo lens] is a contact lens that essentially has wireless power and data transmission for a small micro LED projector that is placed over the center of the eye," explains David Hobbs, Director of Product Management at Mojo Vision. "[It] displays critical heads-up information when you need it and fades into the background when you're ready to continue on with your day."
Eventually, Mojo Visions' technology could replace our beloved smart devices but the first generation of the Mojo smart lens will be used to help the 2.2 billion people globally who suffer from vision impairment.
"If you think of the eye as a camera [for the visually impaired], the sensors are not working properly," explains Dr. Ashley Tuan, Vice President of Medical Devices at Mojo Vision and fellow of the American Academy of Optometry. "For this population, our lens can process the image so the contrast can be enhanced, we can make the image larger, magnify it so that low-vision people can see it or we can make it smaller so they can check their environment." In January of this year, the FDA granted Breakthrough Device Designation to Mojo, allowing them to have early and frequent discussions with the FDA about technical, safety and efficacy topics before clinical trials can be done and certification granted.
For now, Dr. Tuan is one of the few people who has actually worn the Mojo lens. "I put the contact lens on my eye. It was very comfortable like any contact lenses I've worn before," she describes. "The vision is super clear and then when I put on the accessories, suddenly I see Yoda in front of me and I see my vital signs. And then I have my colleague that prepared a beautiful poem that I loved when I was young [and] I was reading the poem with my eyes closed."
At the moment, there are several electronic glasses on the market like Acesight and Nueyes Pro that provide similar solutions for those suffering from visual impairment, but they are large, cumbersome, and highly visible. Mojo lens would be a discreet, more comfortable alternative that offers users more freedom of movement and independence.
"In the case of augmented-reality contact lenses, there could be an opportunity to improve the lives of people with low vision," says Dr. Thomas Steinemann, spokesperson for the American Academy of Ophthalmology and professor of ophthalmology at MetroHealth Medical Center in Cleveland. "There are existing tools for people currently living with low vision—such as digital apps, magnifiers, etc.— but something wearable could provide more flexibility and significantly more aid in day-to-day tasks."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless.
According to Dr. Tuan, the visually impaired often suffer from depression due to their lack of mobility and 70 percent of them are underemployed. "We hope that they can use this device to gain their mobility so they can get that social aspect back in their lives and then, eventually, employment," she explains. "That is our first and most important goal."
But helping those with low visual capabilities is only Mojo lens' first possible medical application; augmented reality is already being used in medicine and is poised to revolutionize the field in the coming decades. For example, Accuvein, a device that uses lasers to provide real-time images of veins, is widely used by nurses and doctors to help with the insertion of needles for IVs and blood tests.
According to the National Center for Biotechnology Information, augmentation of reality has been used in surgery for many years with surgeons using devices such as Google Glass to overlay critical information about their patients into their visual field. Using software like the Holographic Navigation Platform by Scopsis, surgeons can see a mixed-reality overlay that can "show you complicated tumor boundaries, assist with implant placements and guide you along anatomical pathways," its developers say.
However, according to Dr. Tuan, augmented reality headsets have drawbacks in the surgical setting. "The advantage of [Mojo lens] is you don't need to worry about sweating or that the headset or glasses will slide down to your nose," she explains "Also, our lens is designed so that it will understand your intent, so when you don't want the image overlay it will disappear, it will not block your visual field, and when you need it, it will come back at the right time."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless. Possibilities include live translation of sign language for deaf people; helping those with autism to read emotions; and improving doctors' bedside manner by allowing them to fully engage with patients without relying on a computer.
"[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
Furthermore, the lens could be used to monitor health issues. "We have image sensors in the lens right now that point to the world but we can have a camera pointing inside of your eye to your retina," says Dr. Tuan, "[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
For the moment, the future medical applications of the Mojo lens are still theoretical, but the team is confident they can eventually become a reality after going through the proper regulatory review. The company is still in the process of design, prototype and testing of the lens, so they don't know exactly when it will be available for use, but they anticipate shipping the first available products in the next couple of years. Once it does go to market, it will be available by prescription only for those with visual impairments, but the team's goal is to bring it to broader consumer markets pending regulatory clearance.
"We see that right now there's a unique opportunity here for Mojo lens and invisible computing to help to shape what the next decade of technology development looks like," explains David Hobbs. "We can use [the Mojo lens] to better serve us as opposed to us serving technology better."
Schmidt Ocean Institute co-founder Wendy Schmidt is backed by 32 screens in research vessel Falkor's control room where most of the science takes place on the ship, from mapping to live streaming of underwater robotic dives.
WENDY SCHMIDT is a philanthropist and investor who has spent more than a dozen years creating innovative non-profit organizations to solve pressing global environmental and human rights issues. Recognizing the human dependence on sustaining and protecting our planet and its people, Wendy has built organizations that work to educate and advance an understanding of the critical interconnectivity between the land and the sea. Through a combination of grants and investments, Wendy's philanthropic work supports research and science, community organizations, promising leaders, and the development of innovative technologies. Wendy is president of The Schmidt Family Foundation, which she co-founded with her husband Eric in 2006. They also co-founded Schmidt Ocean Institute and Schmidt Futures.
Editors: The pandemic has altered the course of human history and the nature of our daily lives in equal measure. How has it affected the focus of your philanthropy across your organizations? Have any aspects of the crisis in particular been especially galvanizing as you considered where to concentrate your efforts?
Wendy: The COVID-19 pandemic has made the work of our philanthropy more relevant than ever. If anything, the circumstances of this time have validated the focus we have had for nearly 15 years. We support the need for universal access to clean, renewable energy, healthy food systems, and the dignity of human labor and self-determination in a world of interconnected living systems on land and in the Ocean we are only beginning to understand.
When you consider the disproportionate impact of the COVID-19 virus on people who are poorly paid, poorly housed, with poor nutrition and health care, and exposed to unsafe conditions in the workplace—you see clearly how the systems that have been defining how we live, what we eat, who gets healthcare and what impacts the environment around us—need to change.
"This moment has propelled broad movements toward open publication and open sharing of data and samples—something that has always been a core belief in how we support and advance science."
If the pandemic teaches us anything, we learn what resilience looks like, and the essential role for local small businesses including restaurants, farms and ranches, dairies and fish markets in the long term vitality of communities. There is resonance, local economic benefit, and also accountability in these smaller systems, with shorter supply chains and less vertical integration.
The consolidation of vertically integrated business operations for the sake of global efficiency reveals its essential weakness when supply chains break down and the failure to encourage local economic centers leads to intense systemic disruption and the possibility of collapse.
Editors: For scientists, one significant challenge has been figuring out how to continue research, if at all, during this time of isolation and distancing. Yet, your research vessel Falkor, of the Schmidt Ocean Institute, is still on its expedition exploring the Coral Sea Marine Park in Australia—except now there are no scientists onboard. What was the vessel up to before the pandemic hit? Can you tell us more about how they are continuing to conduct research from afar now and how that's going?
Wendy: We have been extremely fortunate at Schmidt Ocean Institute. When the pandemic hit in March, our research vessel, Falkor, was already months into a year-long program to research unexplored deep sea canyons around Australia and at the Great Barrier Reef. We were at sea, with an Australian science group aboard, carrying on with our mission of exploration, discovery and communication, when we happened upon what we believe to be the world's longest animal—a siphonophore about 150 feet long, spiraling out at a depth of about 2100 feet at the end of a deeper dive in the Ningaloo Canyon off Western Australia. It was the kind of wondrous creature we find so often when we conduct ROV dives in the world's Ocean.
For more than two months this year, Falkor was reportedly the only research vessel in the world carrying on active research at sea. Once we were able to dock and return the science party to shore, we resumed our program at sea offering a scheduled set of now land-based scientists in lockdown in Australia the opportunity to conduct research remotely, taking advantage of the vessel's ship to shore communications, high resolution cameras and live streaming video. It's a whole new world, and quite wonderful in its own way.
Editors: Normally, 10–15 scientists would be aboard such a vessel. Is "remote research" via advanced video technology here to stay? Are there any upsides to this "new normal"?
Wendy: Like all things pandemic, remote research is an adaptation for what would normally occur. Since we are putting safety of the crew and guest scientists at the forefront, we're working to build strong remote connections between our crew, land based scientists and the many robotic tools on board Falkor. There's no substitute for in person work, but what we've developed during the current cruise is a pretty good and productive alternative in a crisis. And what's important is that this critical scientific research into the deep sea is able to continue, despite the pandemic on land.
Editors: Speaking of marine expeditions, you've sponsored two XPRIZE competitions focused on ocean health. Do you think challenge prizes could fill gaps of the global COVID-19 response, for example, to manufacture more testing kits, accelerate the delivery of PPE, or incentivize other areas of need?
Wendy: One challenge we are currently facing is that innovations don't have the funding pathway to scale, so promising ideas by entrepreneurs, researchers, and even major companies are being developed too slowly. Challenge prizes help raise awareness for problems we are trying to solve and attract new people to help solve those problems by giving them a pathway to contribute.
One idea might be for philanthropy to pair prizes and challenges with an "advanced market commitment" where the government commits to a purchase order for the innovation if it meets a certain test. That could be deeply impactful for areas like PPE and the production of testing kits.
Editors: COVID-19 testing, especially, has been sorely needed, here in the U.S. and in developing countries as well as low-income communities. That's why we're so intrigued by your Schmidt Science Fellows grantee Hal Holmes and his work to repurpose a new DNA technology to create a portable, mobile test for COVID-19. Can you tell us about that work and how you are supporting it?
Wendy: Our work with Conservation X Labs began years ago when our foundation was the first to support their efforts to develop a handheld DNA barcode sensor to help detect illegally imported and mislabeled seafood and timber products. The device was developed by Hal Holmes, who became one of our Schmidt Science Fellows and is the technical lead on the project, working closely with Conservation X Labs co-founders Alex Deghan and Paul Bunje. Now, with COVID-19, Hal and team have worked with another Schmidt Science Fellow, Fahim Farzardfard, to repurpose the technology—which requires no continuous power source, special training, or a lab—to serve as a mobile testing device for the virus.
The work is going very well, manufacturing is being organized, and distribution agreements with hospitals and government agencies are underway. You could see this device in use within a few months and have testing results within hours instead of days. It could be especially useful in low-income communities and developing countries where access to testing is challenging.
Editors: How is Schmidt Futures involved in the development of information platforms that will offer productive solutions?
Wendy: In addition to the work I've mentioned, we've also funded the development of tech-enabled tools that can help the medical community be better prepared for the ongoing spike of COVID cases. For example, we funded EdX and Learning Agency to develop an online training to help increase the number of medical professionals who can operate ventilators. The first course is being offered by Harvard University, and so far, over 220,000 medical professionals have enrolled. We have also invested in informational platforms that make it easier to contain the spread of the disease, such as our work with Recidiviz to model the impact of COVID-19 in prisons and outline policy steps states could take to limit the spread.
Information platforms can also play a big part pushing forward scientific research into the virus. For example, we've funded the UC Santa Cruz Virus Browser, which allows researchers to examine each piece of the virus and see the proteins it creates, the interactions in the host cell, and — most importantly — almost everything the recent scientific literature has to say about that stretch of the molecule.
Editors: The scale of research collaboration and the speed of innovation today seem unprecedented. The whole science world has turned its attention to combating the pandemic. What positive big-picture trends do you think or hope will persist once the crisis eventually abates?
Wendy: As in many areas, the COVID crisis has accelerated trends in the scientific world that were already well underway. For instance, this moment has propelled broad movements toward open publication and open sharing of data and samples—something that has always been a core belief in how we support and advance science.
We believe collaboration is an essential ingredient for progress in all areas. Early in this pandemic, Schmidt Futures held a virtual gathering of 160 people across 70 organizations in philanthropy, government, and business interested in accelerating research and response to the virus, and thought at the time, it's pretty amazing this kind of thing doesn't go all the time. We are obviously going to go farther together than on our own...
My husband, Eric, has observed that in the past two months, we've all catapulted 10 years forward in our use of technology, so there are trends already underway that are likely accelerated and will become part of the fabric of the post-COVID world—like working remotely; online learning; increased online shopping, even for groceries; telemedicine; increasing use of AI to create smarter delivery systems for healthcare and many other applications in a world that has grown more virtual overnight.
"Our deepest hope is that out of these alarming and uncertain times will come a renewed appreciation for the tools of science, as they help humans to navigate a world of interconnected living systems, of which viruses are a large part."
We fully expect these trends to continue and expand across the sciences, sped up by the pressures of the health crisis. Schmidt Ocean Institute and Schmidt Futures have been pressing in these directions for years, so we are pleased to see the expansions that should help more scientists work productively, together.
Editors: Trying to find the good amid a horrible crisis, are there any other new horizons in science, philanthropy, and/or your own work that could transform our world for the better that you'd like to share?
Wendy: Our deepest hope is that out of these alarming and uncertain times will come a renewed appreciation for the tools of science, as they help humans to navigate a world of interconnected living systems, of which viruses are a large part. The more we investigate the Ocean, the more we look deeply into what lies in our soils and beneath them, the more we realize we do not know, and moreover, how vulnerable humanity is to the forces of the natural world.
Philanthropy has an important role to play in influencing how people perceive our place in the world and understand the impact of human activity on the rest of the planet. I believe it's philanthropy's role to take risks, to invest early in innovative technologies, to lead where governments and industry aren't ready to go yet. We're fortunate at this time to be able to help those working on tools to better diagnose and treat the virus, and to invest in those working to improve information systems, so citizens and policy makers can make better decisions that can reduce impacts on families and institutions.
From all we know, this isn't likely to be the last pandemic the world will see. It's been said that a crisis comes before change, and we would hope that we can play a role in furthering the work to build systems that are resilient—in information, energy, agriculture and in all the ways we work, recreate, and use the precious resources of our planet.
[This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.