Blood Money: Paying for Convalescent Plasma to Treat COVID-19
A bag of plasma that Tom Hanks donated back in April 2020 after his coronavirus infection. (He was not paid to donate.)
Convalescent plasma – first used to treat diphtheria in 1890 – has been dusted off the shelf to treat COVID-19. Does it work? Should we rely strictly on the altruism of donors or should people be paid for it?
The biologic theory is that a person who has recovered from a disease has chemicals in their blood, most likely antibodies, that contributed to their recovery, and transferring those to a person who is sick might aid their recovery. Whole blood won't work because there are too few antibodies in a single unit of blood and the body can hold only so much of it.
Plasma comprises about 55 percent of whole blood and is what's left once you take out the red blood cells that carry oxygen and the white blood cells of the immune system. Most of it is water but the rest is a complex mix of fats, salts, signaling molecules and proteins produced by the immune system, including antibodies.
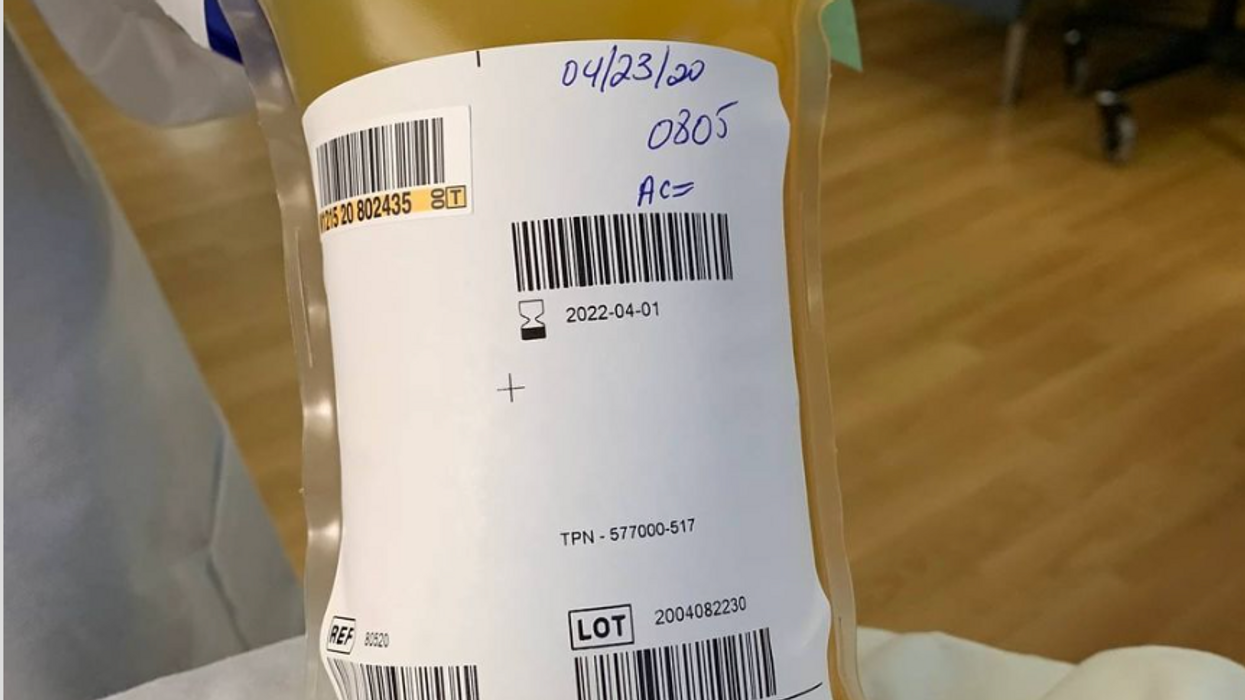
A process called apheresis circulates the donors' blood through a machine that separates out the desired parts of blood and returns the rest to the donor. It takes several times the length of a regular whole blood donation to cycle through enough blood for the process. The end product is a yellowish concentration called convalescent plasma.
Recent History
It was used extensively during the great influenza epidemic off 1918 but fell out of favor with the development of antibiotics. Still, whenever a new disease emerges – SARS, MERS, Ebola, even antibiotic-resistant bacteria – doctors turn to convalescent plasma, often as a stopgap until more effective antibiotic and antiviral drugs are developed. The process is certainly safe when standard procedures for handling blood products are followed, and historically it does seem to be beneficial in at least some patients if administered early enough in the disease.
With few good treatment options for COVID-19, doctors have given convalescent plasma to more than a hundred thousand Americans and tens of thousand of people elsewhere, to mixed results. Placebo-controlled trials could give a clearer picture of plasma's value but it is difficult to enroll patients facing possible death when the flip of a coin will determine who will receive a saline solution or plasma.
And the plasma itself isn't some uniform pill stamped out in a factory, it's a natural product that is shaped by the immune history of the donor's body and its encounter not just with SARS-CoV-2 but a lifetime of exposure to different pathogens.
Researchers believe antibodies in plasma are a key factor in directly fighting the virus. But the variety and quantity of antibodies vary from donor to donor, and even over time from the same donor because once the immune system has cleared the virus from the body, it stops putting out antibodies to fight the virus. Often the quality and quantity of antibodies being given to a patient are not measured, making it somewhat hit or miss, which is why several companies have recently developed monoclonal antibodies, a single type of antibody found in blood that is effective against SARS-CoV-2 and that is multiplied in the lab for use as therapy.
Plasma may also contain other unknown factors that contribute to fighting disease, say perhaps signaling molecules that affect gene expression, which might affect the movement of immune cells, their production of antiviral molecules, or the regulation of inflammation. The complexity and lack of standardization makes it difficult to evaluate what might be working or not with a convalescent plasma treatment. Thus researchers are left with few clues about how to make it more effective.
Industrializing Plasma
Many Americans living along the border with Mexico regularly head south to purchase prescription drugs at a significant discount. Less known is the medical traffic the other way, Mexicans who regularly head north to be paid for plasma donations, which are prohibited in their country; the U.S. allows payment for plasma donations but not whole blood. A typical payment is about $35 for a donation but the sudden demand for convalescent plasma from people who have recovered from COVID-19 commands a premium price, sometimes as high as $200. These donors are part of a fast-growing plasma industry that surpassed $25 billion in 2018. The U.S. supplies about three-quarters of the world's needs for plasma.
Payment for whole blood donation in the U.S. is prohibited, and while payment for plasma is allowed, there is a stigma attached to payment and much plasma is donated for free.
The pharmaceutical industry has shied away from natural products they cannot patent but they have identified simpler components from plasma, such as clotting factors and immunoglobulins, that have been turned into useful drugs from this raw material of plasma. While some companies have retooled to provide convalescent plasma to treat COVID-19, often paying those donors who have recovered a premium of several times the normal rate, most convalescent plasma has come as donations through traditional blood centers.
In April the Mayo Clinic, in cooperation with the FDA, created an expanded access program for convalescent plasma to treat COVID-19. It was meant to reduce the paperwork associated with gaining access to a treatment not yet approved by the FDA for that disease. Initially it was supposed to be for 5000 units but it quickly grew to more than twenty times that size. Michael Joyner, the head of the program, discussed that experience in an extended interview in September.
The Centers for Medicare and Medicaid Services (CMS) also created associated reimbursement codes, which became permanent in August.
Mayo published an analysis of the first 35,000 patients as a preprint in August. It concluded, "The relationships between mortality and both time to plasma transfusion, and antibody levels provide a signature that is consistent with efficacy for the use of convalescent plasma in the treatment of hospitalized COVID-19 patients."
It seemed to work best when given early in infection and in larger doses; a similar pattern has been seen in studies of monoclonal antibodies. A revised version will soon be published in a major medical journal. Some criticized the findings as not being from a randomized clinical trial.
Convalescent plasma is not the only intervention that seems to work better when used earlier in the course of disease. Recently the pharmaceutical company Eli Lilly stopped a clinical trial of a monoclonal antibody in hospitalized COVID-19 patients when it became apparent it wasn't helping. It is continuing trials for patients who are less sick and begin treatment earlier, as well as in persons who have been exposed to the virus but not yet diagnosed as infected, to see if it might prevent infection. In November the FDA eased access to this drug outside of clinical trials, though it is not yet approved for sale.
Show Me the Money
The antibodies that seem to give plasma its curative powers are fragile proteins that the body produces to fight the virus. Production shuts down once the virus is cleared and the remaining antibodies survive only for a few weeks before the levels fade. [Vaccines are used to train immune cells to produce antibodies and other defenses to respond to exposure to future pathogens.] So they can be usefully harvested from a recovered patient for only a few short weeks or months before they decline precipitously. The question becomes, how does one mobilize this resource in that short window of opportunity?
The program run by the Mayo Clinic explains the process and criteria for donating convalescent plasma for COVID-19, as well as links to local blood centers equipped to handle those free donations. Commercial plasma centers also are advertising and paying for donations.
A majority of countries prohibit paying donors for blood or blood products, including India. But an investigation by India Today touted a black market of people willing to donate convalescent plasma for the equivalent of several hundred dollars. Officials vowed to prosecute, saying donations should be selfless.
But that enforcement threat seemed to be undercut when the health minister of the state of Assam declared "plasma donors will get preference in several government schemes including the government job interview." It appeared to be a form of compensation that far surpassed simple cash.
The small city of Rexburg, Idaho, with a population a bit over 50,000, overwhelmingly Mormon and home to a campus of Brigham Young University, at one point had one of the highest per capita rates of COVID-19 in the current wave of infection. Rumors circulated that some students were intentionally trying to become infected so they could later sell their plasma for top dollar, potentially as much as $200 a visit.
Troubled university officials investigated the allegations but could come up with nothing definitive; how does one prove intentionality with such an omnipresent yet elusive virus? They chalked it up to idle chatter, perhaps an urban legend, which might be associated with alcohol use on some other campus.
Doctors, hospitals, and drug companies are all rightly praised for their altruism in the fight against COVID-19, but they also get paid. Payment for whole blood donation in the U.S. is prohibited, and while payment for plasma is allowed, there is a stigma attached to payment and much plasma is donated for free. "Why do we expect the donors [of convalescent plasma] to be the only uncompensated people in the process? It really makes no sense," argues Mark Yarborough, an ethicist at the UC Davis School of Medicine in Sacramento.
"When I was in grad school, two of my closest friends, at least once a week they went and gave plasma. That was their weekend spending money," Yarborough recalls. He says upper and middle-income people may have the luxury of donating blood products but prohibiting people from selling their plasma is a bit paternalistic and doesn't do anything to improve the economic status of poor people.
"Asking people to dedicate two hours a week for an entire year in exchange for cookies and milk is demonstrably asking too much," says Peter Jaworski, an ethicist who teaches at Georgetown University.
He notes that companies that pay plasma donors have much lower total costs than do operations that rely solely on uncompensated donations. The companies have to spend less to recruit and retain donors because they increase payments to encourage regular repeat donations. They are able to more rationally schedule visits to maximize use of expensive apheresis equipment and medical personnel used for the collection.
It seems that COVID-19 has been with us forever, but in reality it is less than a year. We have learned much over that short time, can now better manage the disease, and have lower mortality rates to prove it. Just how much convalescent plasma may have contributed to that remains an open question. Access to vaccines is months away for many people, and even then some people will continue to get sick. Given the lack of proven treatments, it makes sense to keep plasma as part of the mix, and not close the door to any legitimate means to obtain it.
How One University Is Successfully Tackling COVID-19
Alma Mater, a beloved bronze mother figure on the campus of the University of Illinois, Champaign-Urbana, wears a mask to encourage students to do the same as they return for the fall semester. Students are also expected to take a COVID-19 test twice a week.
China, South Korea and other places controlled the SARS-CoV-2 epidemic with the early use of strict lockdown and aggressive electronic contact tracing, monitoring, and enforcement.
The tussles in America over voluntary social distancing and wearing a mask in public suggest that more stringent enforcement methods adopted elsewhere would not work here. But one American university has emerged as a model of tough love pandemic management.
While many universities have become hot spots of COVID-19 infections this fall when students returned to campus, the University of Illinois was an exception. It has gotten the virus under control, at least for the moment, at a rate that is far below the national average and with minimal social disruption. Can the program they implemented work in our broader society?
The Illinois model is a comprehensive one which, as elsewhere, includes masking and social distancing, but it also requires a twice-weekly saliva test for SARS-CoV-2. All students and employees are assigned test days when they swipe their ID card and spit in a plastic tube, which is collected hourly and taken to a campus lab.
There a simplified but highly sensitive PCR genetic test goes through many cycles of amplifying the viral RNA. "Tracking three different viral RNA [genes] gives us very high accuracy," explains Martin Burke, the professor who developed the system and is monitoring its implementation at the University of Illinois Urbana-Champaign. They immediately retest any positive sample to confirm the results, "So we think our false positive rate is extremely low. … The goal is to notify the positive person within 30 minutes of a positive test results becoming known."
Testing everyone so frequently, with a sensitive test that can quickly detect small amounts of the virus soon after infection, and isolating those who test positive before the virus can grow to volumes that make it very infectious helps the Illinois system break the chain of transmission.
"The testing we have done is not a silver bullet, it has to be done in combination with other mitigation measures. Our modeling shows that if you have masks, social distancing, and contact tracing you get a very dramatic, in fact synergistic effect with this combination,' says Burke. "So it really has to be a holistic approach with lots of community engagement in order to make this process successful."
The real teeth of enforcement are that people have to display their health status to gain access to campus facilities. A green check mark over their photo on a college ID phone app means they are good to go but a big red X means they are not current on their testing or have tested positive for the virus. Their ID is inactivated and they cannot enter campus facilities until they become compliant. Burke puts it bluntly; "We stop them from going where they want to go, a measure first used successfully with the pandemic in Wuhan, China.
He says they have learned from their experience and evolved their approach. "We never modeled for people who tested positive to ignore that result and go to or host parties, which could spread the infection." But several students did just that, and a few have been suspended for it.
So the university clamped down on enforcing isolation and now requires some higher risk persons to test three times a week to catch any infections earlier. Since more than 95 percent of new infections were among undergraduates, with no crossover from them to the local community, faculty, or graduate students, they have cut back testing of the latter two groups to just once a week.
About a thousand positive tests results have come back so far but no one has been hospitalized. Part of that likely is because the undergraduate population is largely young and healthy with few risk cofactors. But it may also be that with early identification and isolation, about five percent of dorm rooms have been set aside for that purpose, the person adopts healthier patterns of sleeping and eating that allows the immune system to better fight off the virus.
"But when you compare that to the being able to educate our students, perform research, keep our community thriving, our businesses open, if you add it all up, it's a tremendous return on investment."
The logistics are quite impressive for the campus that in ordinary times is home to more than 50,000 students; a lab capable of churning through 20,000 tests a day, with notification of results within hours, not days as is common elsewhere. And the results are equally impressive. The rate of positive test results blipped up to around 3 percent when undergraduates arrived back on campus but that has plummeted to 0.35 percent for the last seven-day period of testing, a tiny fraction of the rate for the nation as a whole. Much of it can be attributed to the closed environment with limited outside contact that might reintroduce the virus.
Still, even while the campus population has dropped by about a third, they are detecting about 250 new infections a week.
The threat of outside contact adding to the risk is why the university amended the undergraduate school calendar to close for Thanksgiving, hold final classes and exams for the semester online, and not return until February.
It doesn't come cheap. Burke estimates it cost $10 million to set up the program and about the same each semester to operate. "But when you compare that to the being able to educate our students, perform research, keep our community thriving, our businesses open, if you add it all up, it's a tremendous return on investment."
Burke acknowledges that they started with some significant advantages. The community is geographically isolated, an electronically linked ID system was already in place for students and employees, they have the ability to control much activity through access to buildings, and they can expel those who do not conform. He believes their system can translate to similar settings but admits, "A big city is very different from a university community." Still, he believes many of those lessons can be translated to different settings.
An alternative story
However, the situation is very different at the University of Colorado, where new infections have surged since undergraduates returned in late August. Administrators recently switched all classes to online only in an attempt to control the virus.
But that wasn't enough for state authorities who cracked down further, just yesterday declaring a two-week lockdown of all students aged 18 to 22, prohibiting gatherings of any size, indoors or out. Students must stay in their rooms except for essential activities, and if any symptoms develop, report for testing. Fraternities and sororities were targeted as past hot spots of infection.
The police will be actively enforcing the lockdown, and violators can face a penalty of up to 90 days in jail and a $1,000 fine.
Skepticism
Public health largely is based upon an appeal to self-interest and altruism, and voluntary compliance with official guidance. Harm reduction often comes into play when an ideal solution meets resistance and coercion plays only a limited role, as when a person with infectious tuberculosis is not compliant with treatment. Many question whether the medical threat of COVID-19 justifies such a sweeping restriction of individual rights of movement and association imposed on everyone simply because of their age and place of residence as is happening in Colorado.
State and federal courts have begun to strike down as an unconstitutional overreach some of the more restrictive decrees to stay at home or close businesses ordered by state and local officials. What was once tolerated as a few weeks or even a few months of restrictions now seems to stretch without an end in sight, and threatens peoples' livelihoods. In this litigious country it seems only a matter of time before someone will challenge some aspects of the Illinois model or similar programs being set up elsewhere as an infringement of their rights.
"I have real concerns about what we have seen over the course of the past several months in terms of going from not enough testing being available to now having more testing [available] because people don't want to be tested, even when they have symptoms," says Michael Osterholm, a noted expert on pandemic preparedness at the University of Minnesota. "We have some college campuses reporting over fifty percent of the students refusing to be tested or refusing to give any of the contacts that might be followed up on."
Often those who have tested positive for the virus "don't want people to know that they're the potential reason there could be an outbreak in their small social circle," says LaQuandra Nesbitt, public health director for Washington, DC. Stigma is one of the main reasons why only 37% of newly infected people have provided names for contact tracing in D.C., and few offer more than a single name.
"We can't test every single person every single day, we would completely go broke, we would be looking at no other health problems. We're not the NFL," says Monica Gandhi. She is a professor of medicine at the University of California San Francisco and works closely with local health officials. "Just because we have a technology doesn't mean that we have to apply it for every purpose that may be indicated. … We would never dream of mass screening the public for influenza."
"Tests don't solve the problem," she argues. Masking is the most crucial piece for Gandhi, along with social distancing, washing hands regularly, and quarantine when testing positive or in contact with someone who is. Those are the actions that break the ongoing spread of transmission. She does support regular testing in high-risk settings such as nursing homes, inpatients in hospitals, and prisons, and periodic surveys in the general population to better understand where the virus is moving.
Drawing from experience with HIV, Gandhi worries that the stigma of a positive result will drive people away from testing. "Low-income persons will be particularly hesitant to get tested, or to share contact information if they do test positive, if they think they may have to quarantine, not work or gain income." That is why San Francisco initially assisted people in isolation with payment of $1285 for two weeks of isolation and other support as part of a right to health program. And this fall, the State of California passed legislation requiring that large businesses continue to pay employees in quarantine.
Tools for self-protection
The American temperament, decentralization, size, administrative complexity, and sheer cost make it highly unlikely that a coercive one-size-fits-all Illinois approach will ever be rolled out from a university campus to the entire nation. People make different decisions in trading off between safety and personal freedom or autonomy, and many are likely to embrace a rapid, inexpensive self-test if one becomes available, much like a home pregnancy test, to proactively monitor their own health.
OraSure Technologies pioneered the first home test for HIV. It is the only over-the-counter saliva test for HIV approved for sale in the U.S. Results show in about 20 minutes. The company went on to develop versions of this test for hepatitis C and Ebola. Thus it came as no surprise when in April the Department of Health and Human Services awarded it a $710 thousand contract to develop a rapid antigen home test for SARS-CoV-2.
Initial optimization studies for the antigen test showed that a nasal sample rather than an oral one generated better results, OraSure president and CEO Stephen Tang told LeapsMag. A test using a nasal swab is expected to be available later this year while work continues to develop an antibody test that uses saliva. He says, "the fundamental challenge is not only to develop the tests but to get it to scale quickly. That's the only way it's really going to matter." The company has manufacturing capacity to produce 35 million tests a year, with about half for SARS-CoV-2, and will double that capacity in steps within the next twelve months, with all of the increased capacity dedicated to COVID-19.
Initial use will be limited to health care workers and by prescription, but the company hopes to make it available over the counter soon after the FDA finalizes its rules on these types of tests for COVID-19. Importantly, OraSure believes its nasal swab test will be able to meet the current FDA standards for at-home tests. No such tests have yet been approved.
Tang says they envision using a phone app with the test, but that's tied to "the question of our century; who owns the data? If you are an individual buying the test, are you really compelled to report to anybody? If you are an employer and you buy the test and your employees take it, are you then entitled to the information because you're the one administering the test? That's all still being debated as well" by regulators, lawyers, and ethicists.
The price hasn't been set but Tang notes that they have "vast experience" in selling directly to the consumer, physicians, and public health systems in the U.S. and in lower-income companies. "We are very aware of what the economics are and what the need is today. We're trying to make this product as widely available to as many people as possible."
Another tool that may help protect the self-motivated are cell phone apps that alert you to potential exposure to others with the virus. Apple, Google and others have developed versions of the app that all work on the same principle and, miraculously, are compatible between the Apple and Android operating system universes. At first glance they look promising.
The glitch is that where they have been available the longest, only about 15-20 percent of users bother to download it, says Bennett Cyphers, a staff technologist with the Electronic Freedom Foundation (EFF), a nonprofit that advocates for privacy and other concerns in cyberspace. He explains, "If 1 in 10 people have the app installed, then only 1 in 100 interactions between everyone is going to be captured by the app. It scales that way; the fewer people you have, then a really, really small fraction of contacts are actually detected."
It is important to remember that much of public health is not the result of policy but of what people do in their daily lives.
Importantly, about 20 percent of Americans do not own a smart phone with the capacity to handle the app; that percentage is even higher among lower income, less educated, older folks who often are most at risk for suffering a severe case of COVID-19. So the value of this tool is likely to remain largely theoretical.
Divining the future
"It's tough to make predictions, especially about the future," the great baseball sage Yogi Berra is reported to have said. Will the COVID-19 pandemic in the U.S. follow the path of Illinois or Colorado?
The recent past often is no guide to such predictions. France, Spain, and Israel once earned plaudits for early and strict enforcement of lockdowns to control spread of the virus and then eased up on those restrictions. At the same time the world watched with condemnation and fascination as Sweden chose to follow a more laissez faire approach, urging voluntary distancing and masking but no major curtailing of activity.
Today the rates of new infections of COVID-19 in the first three countries have exploded to equal or multiples of the rate in Sweden. Which approach was the correct policy? Most people say it is still too early to tell for sure. The same can be said for the examples of Illinois and Colorado.
And then there is the puzzling example of Manaus, the Brazilian city of 1.8 million in the middle of the Amazon which was slammed with infections as hard as New York City; without the medical infrastructure to cope with the virus, 4000 have died. But then, suddenly, new infections began to taper off, and nobody claims to understand why, it certainly wasn't because official policies changed. One guess is that perhaps the region reached herd immunity, but that is simply speculation.
One can pick and choose examples of tough enforcement of quarantine or none to prove their point for the short term. But draconian measures will not be tolerated for long in a free society, and there is no clear, overwhelming evidence that over the long run one policy approach works better than another.
It is important to remember that much of public health is not the result of policy but of what people do in their daily lives. We have come remarkably far in what is still only months since we first heard the name of the virus. Death rates have fallen dramatically as we have learned how to better manage severe disease, often by adapting treatments for other diseases. And there is reason for optimism with the large number of vaccine candidates already in human trials.
We also have learned that we can control much of our own fate through simple but concerted actions in our daily lives such as social distancing, wearing masks, and washing hands. Let's not only remember those facts, but practice them.
From Crap to Cure: The Story of Fecal Transplants
Meg Newman, who suffered from C. difficile, underwent a fecal transplant that helped restore her to health.
C. difficile had Meg Newman's number; it had struck her 18 different times beginning in 1985. The bacterial infection takes over the gut bringing explosive diarrhea, dehydration, weight loss, and at its worst depletes blood platelets. It causes nearly 30,000 deaths each year in the U.S. alone.
"I was one sick puppy as that point and literally three days after the transplant I was doing pretty well, day four even better."
Meg knew these statistics not just from personal experience but also because she was a doctor at San Francisco General Hospital. Antibiotics had sometimes helped to treat the infection, but it never quite seemed to go away. Now, "It felt like part of my colon was sort of sliding off as I had the bowel movement." On her worst day she counted 33 bowel movements. It was 2005 and she knew she was at the end of her rope.
Medical training had taught Meg to look at the data. So when antibiotics failed, she searched the literature for other options. One was a seemingly off-the-wall treatment called fecal transplants, which essentially gives poop from a healthy person to one who is sick.
Its roots stretch back to "yellow soup" used to treat dysentery in China nearly two thousand years ago, in which ancient Chinese treaters would combine stool with liquid, mash it up, and administer it. The approach also is commonly used in veterinary medicine today. However, there were only about three papers on its use in humans in the medical literature at that time, she recalls. Still, the logic of the intervention appealed to her.
The gut microbiome as a concept and even a word were not widely known fifteen years ago. But the idea that the microbial community in her gut was in disarray, and a transplant of organisms from a healthy gut might help restore a more normal ecology made sense. And besides, the failure of standard medicine left her few options.
Meg spoke with a colleague, gastroenterologist Neil Stollman, about a possible fecal microbial transplant (FMT). Only a handful of doctors in the U.S. had ever done the procedure; Stollman had tried it just once before. After conversation with Newman, he agreed to do it.
They decided on Meg's partner Sherry as the donor. "I try very hard to use intimate sexual partners as the donor," explains Stollman. The reason is to reduce disease risk: "The logic there is pretty straightforward. If you have unprotected sex with this individual, in a monogamous way for a period of time, you have assumed pretty much any infectious risk," like hepatitis, HIV, and syphilis, he says. Other donors would be screened using the same criteria used to screen blood donations, plus screening for parasites that can live in stool but not blood.
The procedure
Martini aficionados fall into two camps, fans of shaken or stirred. In the early days the options for producing of fecal transplants were a blender or hand shaken. Stollman took the hands-on approach, mixing Sherry's fecal donation with saline to create "a milkshake in essence." He filtered it several times through gauze to get out the lumps.
Then he inserted a colonoscope, a long flexible tube, through the anus into Meg's colon. Generally a camera goes through the tube to look for polyps and cancers, while other tools can snip off polyps and retrieve tissue samples. Today he used it to insert the fecal "milkshake" as high up the colon as he could go. Imodium and bed rest were the final pieces. It works about 90 percent of the time today.
Meg went home with fingers crossed. "And within about two weeks things just slowed down; the diarrhea just stopped. I felt better so my appetite was better." The tide had turned, though it would take months to slowly repair the toll taken on her body from disease and antibiotics.
Then in 2011 another serious medical challenge required heavy use of antibiotics and Meg's C. difficile came roaring back; she needed a second FMT. Sherry had a bad sinus infection and had been on antibiotics, so that ruled her out as a donor. Red, Meg's godson, volunteered. He was twenty-one and had little or no exposure to antibiotics, which can harm friendly bacteria living in the gut.
"I was one sick puppy as that point," Meg recalls, "and literally three days after the transplant [from Red] I was doing pretty well, day four even better. It was unbelievable." It illustrated that donors are not the same, and that while an intimate partner may be the safest option, it also may not be the most efficacious donation in terms of providing missing parts of the microbial ecosystem.
Going mainstream
By then, FMTs were starting to come out of the shadows as more than just a medical oddity. One gigantic milestone in changing perceptions was a Dutch study on using the procedure to treat C. difficile that was published in January 2013 in the New England Journal of Medicine. "That was the first trial where people said, look this isn't voodoo. This wasn't made up; it really worked," says Colleen Kelly, another pioneer in using FMTs to treat C. difficile and a researcher at Brown University. A single dose was successful more than 80 percent of the time in resolving disease in patients who had failed multiple regimens of antibiotics.
Charlatans pounced on the growing interest in the microbiome, promoting FMT as a cure for all sorts of ailments for which there was no scientific evidence. The FDA stepped in, announcing it would regulate the procedure as a drug, and essentially banned use of FMTs until it wrote regulations. But the outcry from physicians and patients was so great it was forced to retreat and has allowed continued use in treating C. difficile albeit on an interim regulatory basis that has continued since 2013.
Another major change was the emergence of stool banks, modeled on blood banks. The most widely know is OpenBiome, established in 2012 as a nonprofit by young researchers at Harvard and MIT. It aimed to standardize donation of stool and screening for disease, and package them in frozen form for colonoscopic delivery, or pill form. It greatly simplified the process and more doctors became willing to use FMTs to treat C. difficile. Today, some gastroenterologists specialize in administering the transplants as a feature of their practice.
To be sure, there have been some setbacks, including a transplant between family members where the recipient became obese, likely in part because of bacteria in the material she received. The same thing has occurred in studies in mice. And last year, an elderly man died from a toxic strain of E. coli that was in material provided by a stool bank. That caused the FDA to add that pathogen to the list of those one must screen for in products designed for use as fecal transplants.
Cost remains an issue. OpenBiome charges $1500-$2000 per transplant dose, depending on whether a frozen or pill form of the product is used. And that is likely to go up as the FDA increases the number of diseases that must be screened for, such as the virus that causes COVID-19, which is present in feces and likely can be transmitted through feces. Most insurance companies do not cover FMTs because no product has been formally approved for use by the FDA.
One of the greatest treatment challenges is making the correct diagnosis, says physician Robin Patel, who initially treated patients at the Mayo Clinic in Rochester, Minnesota but now spends most of her time there developing new diagnostics. Many things can cause diarrhea and the simple presence of the organism does not mean that C. difficile is causing it. In addition, many people are colonized with the bug but never develop symptoms of the disease.
Patel used the expensive tool of whole genome sequencing to look in great detail at C. difficile in patients who were treated with antibiotics for the infection and had recurrent diarrhea. "Some of them, as you might predict, were getting their symptoms with the same exact strain [of C. difficile] but others were not, it was a different strain," suggesting that they had been reinfected.
If it is a different strain, you might want to try antibiotics, she says, but if the same strain is present, then you might want to try a different approach such as FMT. Whole genome sequencing is still too slow and expensive to use in regularly treating patients today, but Patel hopes to develop a rapid, cost-effective test to help doctors make those types of decisions.
Biotech companies are trying to develop alternatives to poop as a source for transplant to treat C. difficile. They are picking and choosing different bacteria that they can grow and then combine into a product, to varying degrees of success, but none have yet crossed the finish line of FDA approval.
"I think [the future of FMTs] is going to be targeted, even custom-made."
The FDA would like such a product because it is used to dealing with small molecule drugs that are standardized and produced in batches. Companies are pursing it because, as Kelly says, they are like sharks "smelling money in the water." Approval of such a product might cause the FDA to shut down existing stool banks that now exist in a regulatory limbo, leaving the company with a monopoly of exclusive rights to the treatment.
Back when Meg received her first fecal transplant, the procedure was so obscure that the guidelines for treating C. difficile put out by the American College of Gastroenterology didn't even mention FMT. The procedure crept into the 2013 revision of those guidelines as a treatment of last resort. Guidance under review for release later this year or early next year will ease use further but stop short of making it a first option.
Stollman imagines a future holy grail in treating C. difficile: "You give me a stool specimen and I run it through a scanner that tells me you have too much of this and too little of that. I have a sense of what normal [microbial composition of the gut] should be and add some of this and subtract some of that. Maybe I even give you some antibiotics to get rid of some of the bad guys, give you some probiotics. I think it is going to be targeted, even custom-made."
His complete vision for treating C. difficile won't arrive tomorrow, but given how rapidly FMTs have become part of medicine, it is likely to arrive in pieces and more quickly than one might think.
About five years ago Meg discovered she had an antibody deficiency that contributed to her health problems, including vulnerability to C. difficile. She began supplementation with immunoglobulin and "that has made a huge difference in my health. It is just unbelievable how much better I am." She is grateful that fecal transplants gave her the time to figure that out. She believes "there's every reason to consider it [FMT] as a first-line treatment and do the studies, ASAP."