Flags of America and China illuminated by light bulbs with a question mark between them.
Over the past two millennia, Chinese ingenuity has spawned some of humanity's most consequential inventions. Without gunpowder, guns, bombs, and rockets; without paper, printing, and money printed on paper; and without the compass, which enabled ships to navigate the open ocean, modern civilization might never have been born.
Today, a specter is haunting the developed world: Chinese innovation dominance. And the results have been so spectacular that the United States feels its preeminence threatened.
Yet China lapsed into cultural and technological stagnation during the Qing dynasty, just as the Scientific Revolution was transforming Europe. Western colonial incursions and a series of failed rebellions further sapped the Celestial Empire's capacity for innovation. By the mid-20th century, when the Communist triumph led to a devastating famine and years of bloody political turmoil, practically the only intellectual property China could offer for export was Mao's Little Red Book.
After Deng Xiaoping took power in 1978, launching a transition from a rigidly planned economy to a semi-capitalist one, China's factories began pumping out goods for foreign consumption. Still, originality remained a low priority. The phrase "Made in China" came to be synonymous with "cheap knockoff."
Today, however, a specter is haunting the developed world: Chinese innovation dominance. It first wafted into view in 2006, when the government announced an "indigenous innovation" campaign, dedicated to establishing China as a technology powerhouse by 2020—and a global leader by 2050—as part of its Medium- and Long-Term National Plan for Science and Technology Development. Since then, an array of initiatives have sought to unleash what pundits often call the Chinese "tech dragon," whether in individual industries, such as semiconductors or artificial intelligence, or across the board (as with the Made in China 2025 project, inaugurated in 2015). These efforts draw on a well-stocked bureaucratic arsenal: state-directed financing; strategic mergers and acquisitions; competition policies designed to boost domestic companies and hobble foreign rivals; buy-Chinese procurement policies; cash incentives for companies to file patents; subsidies for academic researchers in favored fields.
The results have been spectacular—so much so that the United States feels its preeminence threatened. Voices across the political spectrum are calling for emergency measures, including a clampdown on technology transfers, capital investment, and Chinese students' ability to study abroad. But are the fears driving such proposals justified?
"We've flipped from thinking China is incapable of anything but imitation to thinking China is about to eat our lunch," says Kaiser Kuo, host of the Sinica podcast at supchina.com, who recently returned to the U.S after 20 years in Beijing—the last six as director of international communications for the tech giant Baidu. Like some other veteran China-watchers, Kuo believes neither extreme reflects reality. "We're in as much danger now of overestimating China's innovative capacity," he warns, "as we were a few years ago of underestimating it."
A Lab and Tech-Business Bonanza
By many measures, China's innovation renaissance is mind-boggling. Spending on research and development as a percentage of gross domestic product nearly quadrupled between 1996 and 2016, from .56 percent to 2.1 percent; during the same period, spending in the United States rose by just .3 percentage points, from 2.44 to 2.79 percent of GDP. China is now second only to the U.S. in total R&D spending, accounting for 21 percent of the global total of $2 trillion, according to a report released in January by the National Science Foundation. In 2016, the number of scientific publications from China exceeded those from the U.S. for the first time, by 426,000 to 409,000. Chinese researchers are blazing new trails on the frontiers of cloning, stem cell medicine, gene editing, and quantum computing. Chinese patent applications have soared from 170,000 to nearly 3 million since 2000; the country now files almost as many international patents as the U.S. and Japan, and more than Germany and South Korea. Between 2008 and 2017, two Chinese tech firms—Huawei and ZTE—traded places as the world's top patent filer in six out of nine years.
"China is still in its Star Trek phase, while we're in our Black Mirror phase." Yet there are formidable barriers to China beating America in the innovation race—or even catching up anytime soon.
Accompanying this lab-based ferment is a tech-business bonanza. China's three biggest internet companies, Baidu, Alibaba Group and Tencent Holdings (known collectively as BAT), have become global titans of search, e-commerce, mobile payments, gaming, and social media. Da-Jiang Innovations in Science and Technology (DJI) controls more than 70 percent of the world's commercial drone market. Of the planet's 262 "unicorns" (startups worth more than a billion dollars), about one-third are Chinese. The country attracted $77 billion in venture capital investment between 2014 and 2016, according to Fortune, and is now among the top three markets for VC in emerging technologies including AI, virtual reality, autonomous vehicles, and 3D printing.
These developments have fueled a buoyant techno-optimism in China that contrasts sharply with the darker view increasingly prevalent in the West—in part, perhaps, because China's historic limits on civil liberties have inured the populace to the intrusive implications of, say, facial recognition technology or social-credit software, which are already being used to tighten government control. "China is still in its Star Trek phase, while we're in our Black Mirror phase," Kuo observes. By contrast with Americans' ambivalent attitudes toward Facebook founder Mark Zuckerberg or Amazon's Jeff Bezos, he adds, most Chinese regard tech entrepreneurs like Baidu's Robin Li and Alibaba's Jack Ma as "flat-out heroes."
Yet there are formidable barriers to China beating America in the innovation race—or even catching up anytime soon. Many are catalogued in The Fat Tech Dragon, a 2017 monograph by Scott Kennedy, deputy director of the Freeman Chair in China Studies and director of the Project on Chinese Business and Political Economy at the Center for Strategic and International Studies. Among the obstacles, Kennedy writes, are "an education system that encourages deference to authority and does not prepare students to be creative and take risks, a financial system that disproportionately funnels funds to undeserving state-owned enterprises… and a market structure where profits can be made through a low-margin, high-volume strategy or through political connections."
China's R&D money, Kennedy points out, is mostly showered on the "D": of the $209 billion spent in 2015, only 5 percent went toward basic research, 10.8 percent toward applied research, and a massive 84.2 percent toward development. While fully half of venture capital in the States goes to early-stage startups, the figure for China is under 20 percent; true "angel" investors are scarce. Likewise, only 21 percent of Chinese patents are for original inventions, as opposed to tweaks of existing technologies. Most problematic, the domestic value of patents in China is strikingly low. In 2015, the country's patent licensing generated revenues of just $1.75 billion, compared to $115 billion for IP licensing in the U.S. in 2012 (the most recent year for which data is available). In short, Kennedy concludes, "China may now be a 'large' IP country, but it is still a 'weak' one."
"[The Chinese] are trying very hard to keep the economy from crashing, but it'll happen eventually. Then there will be a major, major contraction."
Anne Stevenson-Yang, co-founder and research director of J Capital Research, and a leading China analyst, sees another potential stumbling block: the government's obsession with neck-snapping GDP growth. "What China does is to determine, 'Our GDP growth will be X,' and then it generates enough investment to create X," Stevenson-Yang explains. To meet those quotas, officials pour money into gigantic construction projects, creating the empty "ghost cities" that litter the countryside, or subsidize industrial production far beyond realistic demand. "It's the ultimate Ponzi-scheme economy," she says, citing as examples the Chinese cellphone and solar industries, which ballooned on state funding, flooded global markets with dirt-cheap products, thrived just long enough to kill off most of their overseas competitors, and then largely collapsed. Such ventures, Stevenson-Yang notes, have driven China's debt load perilously high. "They're trying very hard to keep the economy from crashing, but it'll happen eventually," she predicts. "Then there will be a major, major contraction."
"An Intensifying Race Toward Techno-Nationalism"
The greatest vulnerability of the Chinese innovation boom may be that it still depends heavily on imported IP. "Over the last few years, China has placed its bets on a combination of global knowledge sourcing and indigenous technology development," says Dieter Ernst, a senior fellow at the Centre for International Governance Innovation in Waterloo, Canada, and the East-West Center in Honolulu, who has served as an Asia advisor for the U.N. and the World Bank. Aside from international journals (and, occasionally, industrial espionage), Chinese labs and corporations obtain non-indigenous knowledge in a number of ways: by paying licensing fees; recruiting Chinese scientists and engineers who've studied or worked abroad; hiring professionals from other countries; or acquiring foreign companies. And though enforcement of IP laws has improved markedly in recent years, foreign businesses are often pressured to provide technology transfers in exchange for access to markets.
Many of China's top tech entrepreneurs—including Ma, Li, and Alibaba's Joseph Tsai—are alumni of U.S. universities, and, as Kuo puts it, "big fans of all things American." Unfortunately, however, Americans are ever less likely to be fans of China, thanks largely to that country's sometimes predatory trade practices—and also to what Ernst calls "an intensifying race toward techno-nationalism." With varying degrees of bellicosity and consistency, leaders of both U.S. parties embrace elements of the trend, as do politicians (and voters) across much of Europe. "There's a growing consensus that China is poised to overtake us," says Ernst, "and that we need to design policies to obstruct its rise."
One of the foremost liberal analysts supporting this view is Lee Branstetter, a professor of economics and public policy at Carnegie Mellon University and former senior economist on President Barack Obama's Council of Economic Advisors. "Over the decades, in a systematic and premeditated fashion, the Chinese government and its state-owned enterprises have worked to extract valuable technology from foreign multinationals, with an explicit goal of eventually displacing those leading multinationals with successful Chinese firms in global markets," Branstetter wrote in a 2017 report to the United States Trade Representative. To combat such "forced transfers," he suggested, laws could be passed empowering foreign governments to investigate coercive requests and block any deemed inappropriate—not just those involving military-related or crucial infrastructure technology, which current statutes cover. Branstetter also called for "sharply" curtailing Chinese students' access to Western graduate programs, as a way to "get policymakers' attention in Beijing" and induce them to play fair.
Similar sentiments are taking hold in Congress, where the Foreign Investment Risk Review Modernization Act—aimed at strengthening the process by which the Committee on Foreign Investment in the United States reviews Chinese acquisition of American technologies—is expected to pass with bipartisan support, though its harsher provisions were softened due to objections from Silicon Valley. The Trump Administration announced in May that it would soon take executive action to curb Chinese investments in U.S. tech firms and otherwise limit access to intellectual property. The State Department, meanwhile, imposed a one-year limit on visas for Chinese grad students in high-tech fields.
Ernst argues that such measures are motivated largely by exaggerated notions of China's ability to reach its ambitious goals, and by the political advantages that fearmongering confers. "If you look at AI, chip design and fabrication, robotics, pharmaceuticals, the gap with the U.S. is huge," he says. "Reducing it will take at least 10 or 15 years."
Cracking down on U.S. tech transfers to Chinese companies, Ernst cautions, will deprive U.S. firms of vital investment capital and spur China to retaliate, cutting off access to the nation's gargantuan markets; it will also push China to forge IP deals with more compliant nations, or revert to outright piracy. And restricting student visas, besides harming U.S. universities that depend on Chinese scholars' billions in tuition, will have a "chilling effect on America's ability to attract to researchers and engineers from all countries."
"It's not a zero-sum game. I don't think China is going to eat our lunch. We can sit down and enjoy lunch together."
America's own science and technology community, Ernst adds, considers it crucial to swap ideas with China's fast-growing pool of talent. The 2017 annual meeting of the Palo Alto-based Association for Advancement of Artificial Intelligence, he notes, featured a nearly equal number of papers by researchers in China and the U.S. Organizers postponed the meeting after discovering that the original date coincided with the Chinese New Year.
China's rising influence on the tech world carries upsides as well as downsides, Scott Kennedy observes. The country's successes in e-commerce, he says, "haven't damaged the global internet sector, but have actually been a spur to additional innovation and progress. By contrast, China's success in solar and wind has decimated the global sectors," due to state-mandated overcapacity. "When Chinese firms win through open competition, the outcome is constructive; when they win through industrial policy and protectionism, the outcome is destructive."
The solution, Kennedy and like-minded experts argue, is to discourage protectionism rather than engage in it, adjusting tech-transfer policy just enough to cope with evolving national-security concerns. Instead of trying to squelch China's innovation explosion, they say, the U.S. should seek ways to spread its potential benefits (as happened in previous eras with Japan and South Korea), and increase America's indigenous investments in tech-related research, education, and job training.
"It's not a zero-sum game," says Kaiser Kuo. "I don't think China is going to eat our lunch. We can sit down and enjoy lunch together."
Digital blockchain concept.
The hacker collective known as the Dark Overlord first surfaced in June 2016, when it advertised more than 600,000 patient files from three U.S. healthcare organizations for sale on the dark web. The group, which also attempted to extort ransom from its victims, soon offered another 9 million records pilfered from health insurance companies and provider networks across the country.
Since 2009, federal regulators have counted nearly 5,000 major data breaches in the United States alone, affecting some 260 million individuals.
Last October, apparently seeking publicity as well as cash, the hackers stole a trove of potentially scandalous data from a celebrity plastic surgery clinic in London—including photos of in-progress genitalia- and breast-enhancement surgeries. "We have TBs [terabytes] of this shit. Databases, names, everything," a gang representative told a reporter. "There are some royal families in here."
Bandits like these are prowling healthcare's digital highways in growing numbers. Since 2009, federal regulators have counted nearly 5,000 major data breaches in the United States alone, affecting some 260 million individuals. Although hacker incidents represent less than 20 percent of the total breaches, they account for almost 80 percent of the affected patients. Such attacks expose patients to potential blackmail or identity theft, enable criminals to commit medical fraud or file false tax returns, and may even allow hostile state actors to sabotage electric grids or other infrastructure by e-mailing employees malware disguised as medical notices. According to the consulting agency Accenture, data theft will cost the healthcare industry $305 billion between 2015 and 2019, with annual totals doubling from $40 billion to $80 billion.
Blockchain could put patients in control of their own data, empowering them to access, share, and even sell their medical information as they see fit.
One possible solution to this crisis involves radically retooling the way healthcare data is stored and shared—by using blockchain, the still-emerging information technology that underlies cryptocurrencies such as Bitcoin. And blockchain-enabled IT systems, boosters say, could do much more than prevent the theft of medical data. Such networks could revolutionize healthcare delivery on many levels, creating efficiencies that would reduce medical errors, improve coordination between providers, drive down costs, and give researchers unprecedented insights into patterns of disease. Perhaps most transformative, blockchain could put patients in control of their own data, empowering them to access, share, and even sell their medical information as they see fit. Widespread adoption could result in "a new kind of healthcare economy, in which data and services are quantifiable and exchangeable, with strong guarantees around both the security and privacy of sensitive information," wrote W. Brian Smith, chief scientist of healthcare-blockchain startup PokitDok, in a recent white paper.
Around the world, entrepreneurs, corporations, and government agencies are hopping aboard the blockchain train. A survey by the IBM Institute for Business Value, released in late 2016, found that 16 percent of healthcare executives in 16 countries planned to begin implementing some form of the technology in the coming year; 90 percent planned to launch a pilot program in the next two years. In 2017, Estonia became the first country to switch its medical-records system to a blockchain-based framework. Great Britain and Dubai are exploring a similar move. Yet in countries with more fragmented health systems, most notably the U.S., the challenges remain formidable. Some of the most advanced healthcare applications envisioned for blockchain, moreover, raise technological and ethical questions whose answers may not arrive anytime soon.
By creating a detailed, comprehensive, and immutable timeline of medical transactions, blockchain-based recordkeeping could help providers gauge a patient's long-term health patterns in a way that's never before been possible.
What Exactly Is Blockchain, Anyway?
To understand the buzz around blockchain, it's necessary to grasp (at least loosely) how the technology works. Ordinary digital recordkeeping systems rely on a central administrator that acts as gatekeeper to a treasury of data; if you can sneak past the guard, you can often gain access to the entire hoard, and your intrusion may go undetected indefinitely. Blockchain, by contrast, employs a network of synchronized, replicated databases. Information is scattered among these nodes, rather than on a single server, and is exchanged through encrypted, peer-to-peer pathways. Each transaction is visible to every computer on the network, and must be approved by a majority in order to be successfully completed. Each batch of transactions, or "block," is date- and time-stamped, marked with the user's identity, and given a cryptographic code, which is posted to every node. These blocks form a "chain," preserved in an electronic ledger, that can be read by all users but can't be edited. Any unauthorized access, or attempt at tampering, can be quickly neutralized by these overlapping safeguards. Even if a hacker managed to break into the system, penetrating deeply would be extraordinarily difficult.
Because blockchain technology shares transaction records throughout a network, it could eliminate communication bottlenecks between different components of the healthcare system (primary care physicians, specialists, nurses, and so on). And because blockchain-based systems are designed to incorporate programs known as "smart contracts," which automate functions previously requiring human intervention, they could reduce dangerous slipups as well as tedious and costly paperwork. For example, when a patient gets a checkup, sees a specialist, and fills a prescription, all these actions could be automatically recorded on his or her electronic health record (EHR), checked for errors, submitted for billing, and entered on insurance claims—which could be adjudicated and reimbursed automatically as well. "Blockchain has the potential to remove a lot of intermediaries from existing workflows, whether digital or nondigital," says Kamaljit Behera, an industry analyst for the consulting firm Frost & Sullivan.
The possible upsides don't end there. By creating a detailed, comprehensive, and immutable timeline of medical transactions, blockchain-based recordkeeping could help providers gauge a patient's long-term health patterns in a way that's never before been possible. In addition to data entered by their caregivers, individuals could use app-based technologies or wearables to transmit other information to their records, such as diet, exercise, and sleep patterns, adding new depth to their medical portraits.
Many experts expect healthcare blockchain to take root more slowly in the U.S. than in nations with government-run national health services.
Smart contracts could also allow patients to specify who has access to their data. "If you get an MRI and want your orthopedist to see it, you can add him to your network instead of carrying a CD into his office," explains Andrew Lippman, associate director of the MIT Media Lab, who helped create a prototype healthcare blockchain system called MedRec that's currently being tested at Beth Israel Deaconess Hospital in Boston. "Or you might make a smart contract to allow your son or daughter to access your healthcare records if something happens to you." Another option: permitting researchers to analyze your data for scientific purposes, whether anonymously or with your name attached.
The Recent History, and Looking Ahead
Over the past two years, a crowd of startups has begun vying for a piece of the emerging healthcare blockchain market. Some, like PokitDok and Atlanta-based Patientory, plan to mint proprietary cryptocurrencies, which investors can buy in lieu of stock, medical providers may earn as a reward for achieving better outcomes, and patients might score for meeting wellness goals or participating in clinical trials. (Patientory's initial coin offering, or ICO, raised more than $7 million in three days.) Several fledgling healthcare-blockchain companies have found powerful corporate partners: Intel for Silicon Valley's PokitDok, Kaiser Permanente for Patientory, Philips for Los Angeles-based Gem Health. At least one established provider network, Change Healthcare, is developing blockchain-based systems of its own. Two months ago, Change launched what it calls the first "enterprise-scale" blockchain network in U.S. healthcare—a system to track insurance claim submissions and remittances.
No one, however, has set a roll-out date for a full-blown, blockchain-based EHR system in this country. "We have yet to see anything move from the pilot phase to some kind of production status," says Debbie Bucci, an IT architect in the federal government's Office of the National Coordinator for Health Information Technology. Indeed, many experts expect healthcare blockchain to take root more slowly here than in nations with government-run national health services. In America, a typical patient may have dealings with a family doctor who keeps everything on paper, an assortment of hospitals that use different EHR systems, and an insurer whose system for processing claims is separate from that of the healthcare providers. To help bridge these gaps, a consortium called the Hyperledger Healthcare Working Group (which includes many of the leading players in the field) is developing standard protocols for blockchain interoperability and other functions. Adding to the complexity is the federal Health Insurance and Portability Act (HIPAA), which governs who can access patient data and under what circumstances. "Healthcare blockchain is in a very nascent stage," says Behera. "Coming up with regulations and other guidelines, and achieving large-scale implementation, will take some time."
The ethical implications of buying and selling personal genomic data in an electronic marketplace are doubtless open to debate.
How long? Behera, like other analysts, estimates that relatively simple applications, such as revenue-cycle management systems, could become commonplace in the next five years. More ambitious efforts might reach fruition in a decade or so. But once the infrastructure for healthcare blockchain is fully established, its uses could go far beyond keeping better EHRs.
A handful of scientists and entrepreneurs are already working to develop one visionary application: managing genomic data. Last month, Harvard University geneticist George Church—one of the most influential figures in his discipline—launched a business called Nebula Genomics. It aims to set up an exchange in which individuals can use "Neptune tokens" to purchase DNA sequencing, which will be stored in the company's blockchain-based system; research groups will be able to pay clients for their data using the same cryptocurrency. Luna DNA, founded by a team of biotech veterans in San Diego, plans a similar service, as does a Moscow-based startup called the Zenome Project.
Hossein Rahnama, CEO of the mobile-tech company Flybits and director of research at the Ryerson Centre for Cloud and Context-Aware Computing in Toronto, envisions a more personalized way of sharing genomic data via blockchain. His firm is working with a U.S. insurance company to develop a service that would allow clients in their 20s and 30s to connect with people in their 70s or 80s with similar genomes. The young clients would learn how the elders' lifestyle choices had influenced their health, so that they could modify their own habits accordingly. "It's intergenerational wisdom-sharing," explains Rahnama, who is 38. "I would actually pay to be a part of that network."
The ethical implications of buying and selling personal genomic data in an electronic marketplace are doubtless open to debate. Such commerce could greatly expand the pool of subjects for research in many areas of medicine, enabling the kinds of breakthroughs that only Big Data can provide. Yet it could also lead millions to surrender the most private information of all—the secrets of their cells—to buyers with less benign intentions. The Dark Overlord, one might argue, could not hope for a more satisfying victory.
These scenarios, however, are pure conjecture. After the first web page was posted, in 1991, Lippman observes, "a whole universe developed that you couldn't have imagined on Day 1." The same, he adds, is likely true for healthcare blockchain. "Our vision is to make medical records useful for you and for society, and to give you more control over your own identity. Time will tell."
Can Spare Parts from Pigs Solve Our Organ Shortage?
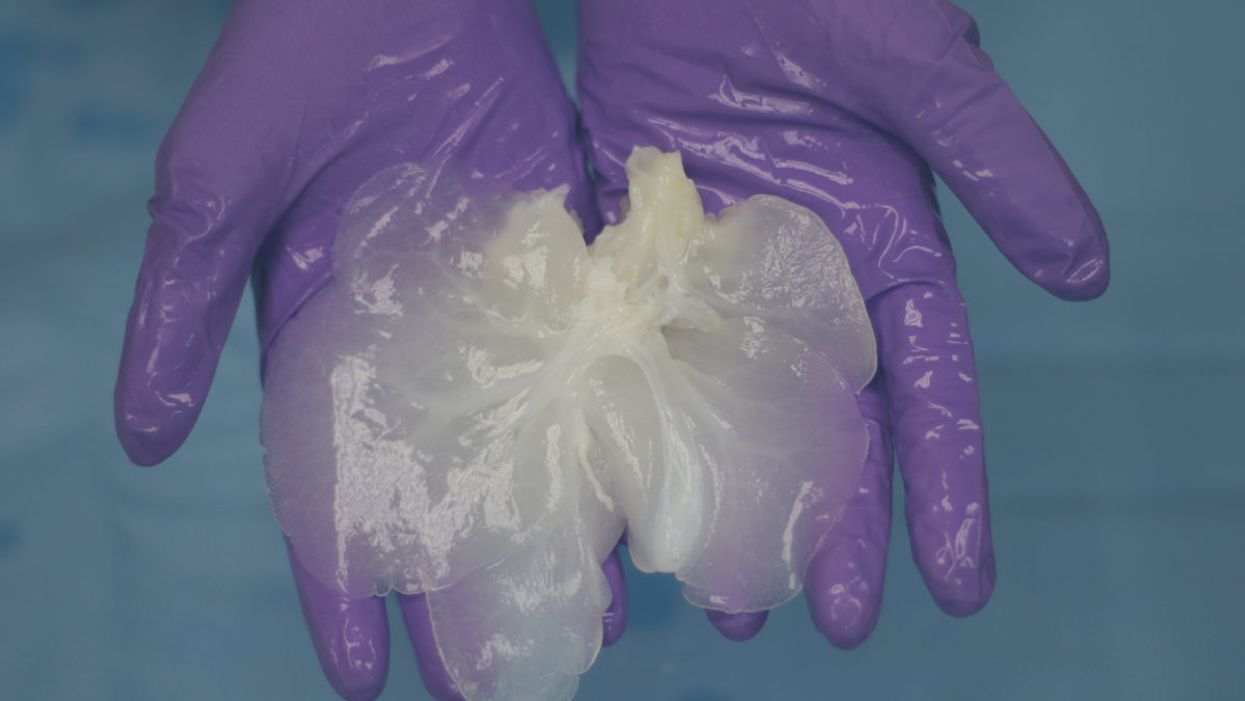
A decellularized small porcine liver.
Jennifer Cisneros was 18 years old, commuting to college from her family's home outside Annapolis, Maryland, when she came down with what she thought was the flu. Over the following weeks, however, her fatigue and nausea worsened, and her weight began to plummet. Alarmed, her mother took her to see a pediatrician. "When I came back with the urine cup, it was orange," Cisneros recalls. "He was like, 'Oh, my God. I've got to send you for blood work.'"
"Eventually, we'll be better off than with a human organ."
Further tests showed that her kidneys were failing, and at Johns Hopkins Hospital, a biopsy revealed the cause: Goodpasture syndrome (GPS), a rare autoimmune disease that attacks the kidneys or lungs. Cisneros was put on dialysis to filter out the waste products that her body could no longer process, and given chemotherapy and steroids to suppress her haywire immune system.
The treatment drove her GPS into remission, but her kidneys were beyond saving. At 19, Cisneros received a transplant, with her mother as donor. Soon, she'd recovered enough to return to school; she did some traveling, and even took up skydiving and parasailing. Then, after less than two years, rejection set in, and the kidney had to be removed.
She went back on dialysis until she was 26, when a stranger learned of her plight and volunteered to donate. That kidney lasted four years, but gave out after a viral infection. Since 2015, Cisneros—now 32, and working as an office administrator between thrice-weekly blood-filtering sessions—has been waiting for a replacement.
She's got plenty of company. About 116,000 people in the United States currently need organ transplants, but fewer than 35,000 organs become available every year. On average, 20 people on the waiting list die each day. And despite repeated campaigns to boost donorship, the gap shows no sign of narrowing.
"This is going to revolutionize medicine, in ways we probably can't yet appreciate."
For decades, doctors and scientists have envisioned a radical solution to the shortage: harvesting other species for spare parts. Xenotransplantation, as the practice is known, could provide an unlimited supply of lifesaving organs for patients like Cisneros. Those organs, moreover, could be altered by genetic engineering or other methods to reduce the danger of rejection—and thus to eliminate the need for immunosuppressive drugs, whose potential side effects include infections, diabetes, and cancer. "Eventually, we'll be better off than with a human organ," says David Cooper, MD, PhD, co-director of the xenotransplant program at the University of Alabama School of Medicine. "This is going to revolutionize medicine, in ways we probably can't yet appreciate."
Recently, progress toward that revolution has accelerated sharply. The cascade of advances began in April 2016, when researchers at the National Heart, Lung, and Blood Institute (NHLBI) reported keeping pig hearts beating in the abdomens of five baboons for a record-breaking mean of 433 days, with one lasting more than two-and-a-half years. Then a team at Emory University announced that a pig kidney sustained a rhesus monkey for 435 days before being rejected, nearly doubling the previous record. At the University of Munich, in Germany, researchers doubled the record for a life-sustaining pig heart transplant in a baboon (replacing the animal's own heart) to 90 days. Investigators at the Salk Institute and the University of California, Davis, declared that they'd grown tissue in pig embryos using human stem cells—a first step toward cultivating personalized replacement organs. The list goes on.
Such breakthroughs, along with a surge of cash from biotech investors, have propelled a wave of bullish media coverage. Yet this isn't the first time that xenotransplantation has been touted as the next big thing. Twenty years ago, the field seemed poised to overcome its final hurdles, only to encounter a setback from which it is just now recovering.
Which raises a question: Is the current excitement justified? Or is the hype again outrunning the science?
A History of Setbacks
The idea behind xenotransplantation dates back at least as far as the 17th century, when French physician Jean-Baptiste Denys tapped the veins of sheep and cows to perform the first documented human blood transfusions. (The practice was banned after two of the four patients died, probably from an immune reaction.) In the 19th century, surgeons began transplanting corneas from pigs and other animals into humans, and using skin xenografts to aid in wound healing; despite claims of miraculous cures, medical historians believe those efforts were mostly futile. In the 1920s and '30s, thousands of men sought renewed vigor through testicular implants from monkeys or goats, but the fad collapsed after studies showed the effects to be imaginary.
Research shut down when scientists discovered a virus in pig DNA that could infect human cells.
After the first successful human organ transplant in 1954—of a kidney, passed between identical twin sisters—the limited supply of donor organs brought a resurgence of interest in animal sources. Attention focused on nonhuman primates, our species' closest evolutionary relatives. At Tulane University, surgeon Keith Reemstma performed the first chimpanzee-to-human kidney transplants in 1963 and '64. Although one of the 13 patients lived for nine months, the rest died within a few weeks due to organ rejection or infections. Other surgeons attempted liver and heart xenotransplants, with similar results. Even the advent of the first immunosuppressant drug, cyclosporine, in 1983, did little to improve survival rates.
In the 1980s, Cooper—a pioneering heart transplant surgeon who'd embraced the dream of xenotransplantation—began arguing that apes and monkeys might not be the best donor animals after all. "First of all, there's not enough of them," he explains. "They breed in ones and twos, and take years to grow to full size. Even then, their hearts aren't big enough for a 70-kg. patient." Pigs, he suggested, would be a more practical alternative. But when he tried transplanting pig organs into nonhuman primates (as surrogates for human recipients), they were rejected within minutes.
In 1992, Cooper's team identified a sugar on the surface of porcine cells, called alpha-1,3-galactose (a-gal), as the main target for the immune system's attack. By then, the first genetically modified pigs had appeared, and biotech companies—led by the Swiss-based pharma giant Novartis—began pouring millions of dollars into developing one whose organs could elude or resist the human body's defenses.
Disaster struck five years later, when scientists reported that a virus whose genetic code was written into pig DNA could infect human cells in lab experiments. Although there was no evidence that the virus, known as PERV (for porcine endogenous retrovirus) could cause disease in people, the discovery stirred fears that xenotransplants might unleash a deadly epidemic. Facing scrutiny from government regulators and protests from anti-GMO and animal-rights activists, Novartis "pulled out completely," Cooper recalls. "They slaughtered all their pigs and closed down their research facility." Competitors soon followed suit.
The riddles surrounding animal-to-human transplants are far from fully solved.
A New Chapter – With New Questions
Yet xenotransplantation's visionaries labored on, aided by advances in genetic engineering and immunosuppression, as well as in the scientific understanding of rejection. In 2003, a team led by Cooper's longtime colleague David Sachs, at Harvard Medical School, developed a pig lacking the gene for a-gal; over the next few years, other scientists knocked out genes expressing two more problematic sugars. In 2013, Muhammad Mohiuddin, then chief of the transplantation section at the NHLBI, further modified a group of triple-knockout pigs, adding genes that code for two human proteins: one that shields cells from attack by an immune mechanism known as the complement system; another that prevents harmful coagulation. (It was those pigs whose hearts recently broke survival records when transplanted into baboon bellies. Mohiuddin has since become director of xenoheart transplantation at the University of Maryland's new Center for Cardiac Xenotransplantation Research.) And in August 2017, researchers at Harvard Medical School, led by George Church and Luhan Yang, announced that they'd used CRISPR-cas9—an ultra-efficient new gene-editing technique—to disable 62 PERV genes in fetal pig cells, from which they then created cloned embryos. Of the 37 piglets born from this experiment, none showed any trace of the virus.
Still, the riddles surrounding animal-to-human transplants are far from fully solved. One open question is what further genetic manipulations will be necessary to eliminate all rejection. "No one is so naïve as to think, 'Oh, we know all the genes—let's put them in and we are done,'" biologist Sean Stevens, another leading researcher, told the The New York Times. "It's an iterative process, and no one that I know can say whether we will do two, or five, or 100 iterations." Adding traits can be dangerous as well; pigs engineered to express multiple anticoagulation proteins, for example, often die of bleeding disorders. "We're still finding out how many you can do, and what levels are acceptable," says Cooper.
Another question is whether PERV really needs to be disabled. Cooper and some of his colleagues note that pig tissue has long been used for various purposes, such as artificial heart valves and wound-repair products, without incident; requiring the virus to be eliminated, they argue, will unnecessarily slow progress toward creating viable xenotransplant organs and the animals that can provide them. Others disagree. "You cannot do anything with pig organs if you do not remove them," insists bioethicist Jeantine Lunshof, who works with Church and Yang at Harvard. "The risk is simply too big."
"We've removed the cells, so we don't have to worry about latent viruses."
Meanwhile, over the past decade, other approaches to xenotransplantation have emerged. One is interspecies blastocyst complementation, which could produce organs genetically identical to the recipient's tissues. In this method, genes that produce a particular organ are knocked out in the donor animal's embryo. The embryo is then injected with pluripotent stem cells made from the tissue of the intended recipient. The stem cells move in to fill the void, creating a functioning organ. This technique has been used to create mouse pancreases in rats, which were then successfully transplanted into mice. But the human-pig "chimeras" recently created by scientists were destroyed after 28 days, and no one plans to bring such an embryo to term anytime soon. "The problem is that cells don't stay put; they move around," explains Father Kevin FitzGerald, a bioethicist at Georgetown University. "If human cells wind up in a pig's brain, that leads to a really interesting conundrum. What if it's self-aware? Are you going to kill it?"
Much further along, and less ethically fraught, is a technique in which decellularized pig organs act as a scaffold for human cells. A Minnesota-based company called Miromatrix Medical is working with Mayo Clinic researchers to develop this method. First, a mild detergent is pumped through the organ, washing away all cellular material. The remaining structure, composed mainly of collagen, is placed in a bioreactor, where it's seeded with human cells. In theory, each type of cell that normally populates the organ will migrate to its proper place (a process that naturally occurs during fetal development, though it remains poorly understood). One potential advantage of this system is that it doesn't require genetically modified pigs; nor will the animals have to be raised under controlled conditions to avoid exposure to transmissible pathogens. Instead, the organs can be collected from ordinary slaughterhouses.

Recellularized livers in bioreactors
(Courtesy of Miromatrix)
"We've removed the cells, so we don't have to worry about latent viruses," explains CEO Jeff Ross, who describes his future product as a bioengineered human organ rather than a xeno-organ. That makes PERV a nonissue. To shorten the pathway to approval by the Food and Drug Administration, the replacement cells will initially come from human organs not suitable for transplant. But eventually, they'll be taken from the recipient (as in blastocyst complementation), which should eliminate the need for immunosuppression.
Clinical trials in xenotransplantation may begin as early as 2020.
Miromatrix plans to offer livers first, followed by kidneys, hearts, and eventually lungs and pancreases. The company recently succeeded in seeding several decellularized pig livers with human and porcine endothelial cells, which flocked obediently to the blood vessels. Transplanted into young pigs, the organs showed unimpaired circulation, with no sign of clotting. The next step is to feed all four liver cell types back into decellularized livers, and see if the transplanted organs will keep recipient pigs alive.
Ross hopes to launch clinical trials by 2020, and several other groups (including Cooper's, which plans to start with kidneys) envision a similar timeline. Investors seem to share their confidence. The biggest backer of xenotransplantation efforts is United Therapeutics, whose founder and co-CEO, Martine Rothblatt, has a daughter with a lung condition that may someday require a transplant; since 2011, the biotech firm has poured at least $100 million into companies pursuing such technologies, while supporting research by Cooper, Mohiuddin, and other leaders in the field. Church and Yang, at Harvard, have formed their own company, eGenesis, bringing in a reported $40 million in funding; Miromatrix has raised a comparable amount.
It's impossible to predict who will win the xenotransplantation race, or whether some new obstacle will stop the competition in its tracks. But Jennifer Cisneros is rooting for all the contestants. "These technologies could save my life," she says. If she hasn't found another kidney before trials begin, she has just one request: "Sign me up."