Vaccine Passports Are a Premature Solution to A Challenging Problem
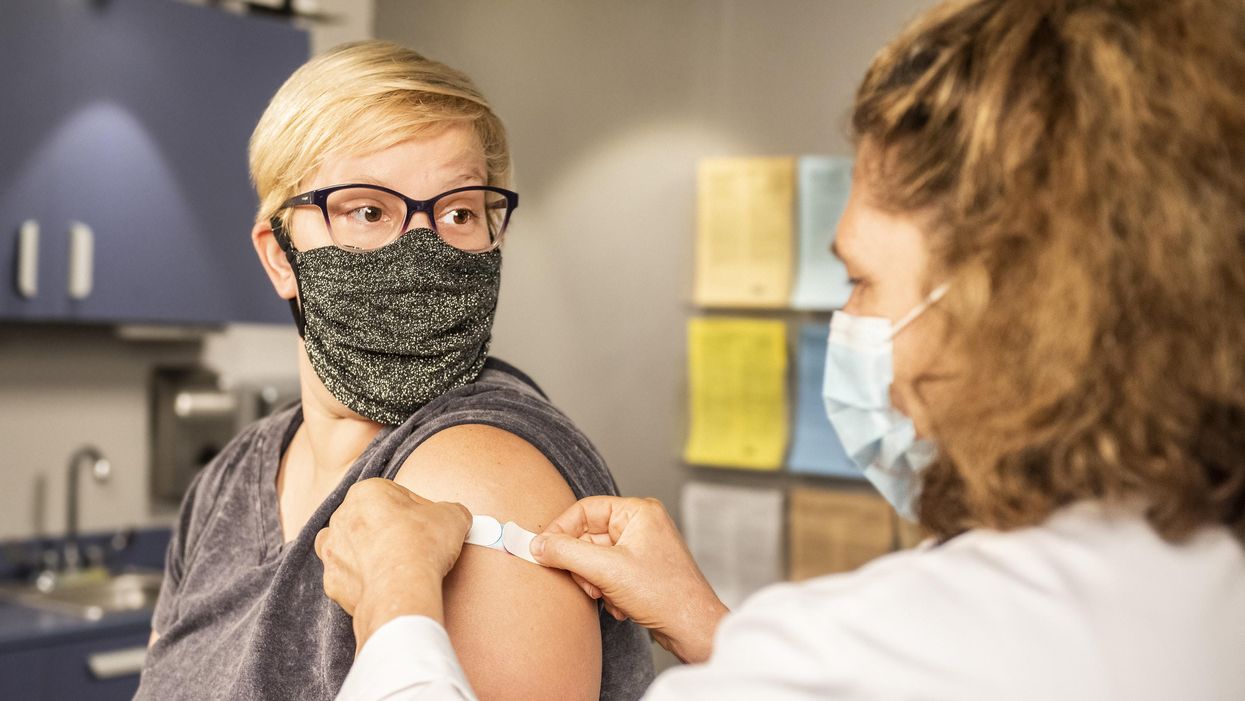
Vaccine passports, if required by businesses and universities, may give people a false sense of security, one expert cautions.
Vaccines are one of the greatest public health accomplishments of all time. For centuries, public health has relied on vaccinations to prevent and control disease outbreaks for a plethora of infectious scourges, with our crowning achievement being the successful eradication of smallpox.
The purpose of vaccine documentation is to provide proof of an individual's protection from either becoming infected or transmitting a vaccine-preventable disease. Vouching for these protections requires a firm knowledge about the epidemiology of the disease, as well as scientific knowledge concerning the efficacy of the vaccine. The vaccines we currently require be documented have met these tests; the vaccine for COVID-19 has not yet been proven to do so.
Let's acknowledge that the term "vaccine passport" is a poor choice of words. Passports are a legal travel document created by nations and governed by law for identification of the bearer to control entry and exit from nation states. They often serve as legal forms of identification and as a record of international travel. They are generally very sophisticated documents that have been created in a secure manner and may include a range of electronic and, in some cases, biometric measures such as fingerprints to ensure the holder is indeed who they say they are. Vaccine passports are medical documents used to document the vaccination status of an individual. They do not undergo the same level of administrative scrutiny and cannot be used to verify that the presenter is indeed the vaccinated individual. Some companies do have electronic methods to address concerns about verification; however, most people currently have paper records that can be easily falsified.
"Vaccine passports" as currently proposed risk giving people a false sense of security.
Successful disease control from vaccination programs relies on the ability to vaccinate at a level that prevents large-scale disease spread and the ability to rapidly identify the presence of disease outbreaks. It requires reliable, safe, and effective vaccines that are easily delivered in clinical and nonclinical settings. Keeping vaccination information as a part of the medical record, and even having a separate specialized vaccine record for personal use, is a time-honored tradition.
Keeping a vaccination record provides a method to keep track of the many shots one receives and serves as a visual reminder to help ensure the appropriate vaccine shot schedule is maintained for vaccines requiring multiple doses. The vaccine record, when combined with vaccine safety monitoring systems, serves as a mechanism to track adverse events to monitor and ensure the safety of vaccines as a consumer product. The record also serves as the official record of vaccination when required for administrative or legally prescribed purposes.
"Vaccine passports" as currently proposed risk giving people a false sense of security. In the case of the COVID-19 vaccines currently approved for use, many of the essential questions remain unanswered. While we do know the current three vaccines are highly protective against severe disease and death, and there is some evidence that these vaccinations do reduce infections and virus transmission of SARS-CoV-2, we do not yet know the full degree to which this occurs.
For example, we know there have been some cases of people that have been infected in close proximity to getting their full vaccination and rare cases of breakthrough reinfections. A breakthrough infection in a restaurant is a challenge for contact tracing, but an outbreak from a movie theater exposure or a baseball game could spark a major outbreak at our current level of vaccination. Current CDC guidance recommends continued mask wearing in order to address these concerns.
We also do not yet know how long the protections will last and if or when a booster or revaccination is required. In effect, it is too soon to know. Should an annual booster shot be required, then a vaccine passport would require annual updating, a process more frequent than renewal of a driver's license.
We also know that the current SARS-CoV-2 virus is mutating briskly. While the current approved vaccines have remained effective overall, there is evidence of some degree of degradation in vaccine effectiveness against some of the circulating strains. We also have sparse data on many of the other emerging strains of concern because we have not had the surveillance capacity in the U.S. to gain an adequate sense of how the virus is changing to fully align vaccine effectiveness with viral capabilities.
The risk of people misusing these "passports" is troubling. The potential for using these documents for hiring, firing or job limitation is a serious concern. Unvaccinated workers are at risk of this form of discrimination even from well-meaning employers or supervisors. Health insurers are prohibited by the Affordable Care Act from discriminating based on preexisting conditions, but they could probably charge a higher premium for unvaccinated individuals. There also is a risk of stigmatizing individuals who are not vaccinated or have left their vaccine documentation at home. Another concern: the opportunity to discriminate based on race, gender, sexual orientation, or religion, using one's vaccination status as an excuse.
These "passports" are being discussed as a "ticket verification" for entry to many activities, including dining at restaurants, flying domestically and/or internationally, going to movie theaters and sporting events, etc. These are all activities we already are doing at reduced levels and for which wearing a mask, hand hygiene and physical distancing are effective disease control practices. COVID-19 vaccines are indeed the measure that will make the ability to totally reopen our society complete, but we are not there yet. Documentation of one's COVID-19 vaccine status may be useful in selected situations in the future. That remains to be seen.
Finally, inadequate vaccine supply and disparities in vaccine delivery have created enormous challenges in providing equal access to vaccination. Also, the amount of misinformation, disinformation, and lingering vaccine hesitancy continue to limit the speed at which we will reach the level of vaccination of the population that would make this documentation meaningful. The requirement for "vaccine passports" is already alienating people who are opposed to vaccinations for a variety of reasons, paradoxically risking reduced vaccine uptake. This politicization of the vaccination effort is of concern. There are indeed people who, due to medical contraindications or legal exemptions, will not be vaccinated, and we do not yet have a national framework on how to address this.
Vaccine passports are not the solution for reopening our society — a robust vaccination program is. The requirement to document one's vaccination status for COVID-19 may one day have its place. For now, it is an idea whose time has not yet come.
Editor's Note: This op/ed is part of a "Big Question" series on the ethics of vaccine passports. Read the flip side argument here.
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

