Vaccines Are the Safest Medical Procedure We Have. Make Your Wager Wisely.
Frontline infectious disease physician Amesh Adalja received his COVID-19 vaccine on December 18th, 2020 in Butler, PA.
In the late 1650's the French polymath and renowned scientist Blaise Pascal, having undergone a religious experience that transformed him into something of a zealot, suggested the following logical strategy regarding belief in God: If there is a God, then believing in him will ensure you an eternity of bliss, while not believing in him could earn you an eternal sentence to misery.
On the other hand, if there is no God, believing in him anyway will cost you very little, and not believing in him will mean nothing in the non-existent after life. Therefore, the only sensible bet is to believe in God. This has come to be known as Pascal's wager.
It has a surprising number of applications beyond concerns for a comfortable afterlife. There are many things for which the value of believing something or not can be seen as a cost vs. likely benefit wager, often without regard to the actual truth of the matter. Since science does not profess to have a final truth, and in many areas freely admits its incomplete knowledge, Pascal's wager can provide a useful method of deciding between two alternatives.
For example, it seems that a significant percentage of the population is suspicious of science, or so we are told. We often hear that some large number, approaching or exceeding half of Americans, do not believe in evolution. This seems remarkable on the face of it because there is no viable scientific opposition to evolution and it is widely accepted by biologists and other life-scientists as being fundamental to understanding biology – from genetics to medicine.
What we are not often told is that most of those who answer negatively about believing in evolution nonetheless understand evolution – or at least the basics of it. They are not stupid, ignorant or uninformed. They have simply made a Pascalian wager. What benefit we might ask is derived from believing in evolution rather than a divine creation? Unless you are a professional biologist it is hard to see how this would affect your everyday life. On the other hand professing a belief in Darwinian evolution over the biblical narrative will likely ostracize you from family, friends, co-workers, your church community - in short most of your social infrastructure. Place your bets.
Can we apply any of this to decisions over the current controversy surrounding vaccination – and in particular the newly arrived Covid-19 vaccine?
While it is true that for entirely economic reasons, this is the first vaccine to be produced in this way, the method is not really new and the science that makes it possible has been developing over the last 40 years.
Common Concerns
There are certainly reasons to be concerned about being vaccinated and it would be a gross over-simplification to consider anyone who expresses reticence about taking a vaccine, this new vaccine in particular, as being just plain dumb or scientifically illiterate or gullible. They need be none of these things and still may be suspicious of the vaccine.
One issue is safety. The vaccine, any vaccine, is designed to mobilize your immune system, essentially to fool it into believing that there is an invading virus present and to mount an immune response. That way it will be ready when the real invasion comes, if it comes. This seems pretty sensible and preferable to going to war with an opponent you know nothing about. But still, it is fooling around with Mother Nature and some people are uneasy about that. Although it must be pointed out that the virus is not at all shy about fooling around with your immune system and many other parts of you, so letting it have its way is not good policy either.
What about a vaccine made of genes? This vaccine is being produced by what is being touted as a new method using RNA – genes. While it is true that for entirely economic reasons, this is the first vaccine to be produced in this way, the method is not really new and the science that makes it possible has been developing over the last 40 years. So it's not so radical as the press makes it seem.
But it is true that this method uses RNA, genetic material, to make the vaccine. We hear a lot about gene modification and the potential dangers associated with it. Why then am I going to allow RNA, genes, to be injected into me? The first thing to realize is that this is exactly what the virus does – so whether you get a vaccine or an infection, you are getting genes injected into you. The virus RNA encodes around 12 functional genes (by comparison humans and other mammals have around 25,000 genes). The virus only contains the genes to make a new virus – it does not have any of the capabilities of a normal cell to actually turn those genes into the proteins that make up the complete virus. It hijacks your cells to do this – and that's how it sickens you, by forcing your cells to make new viruses instead of what they should be doing.
Now the new vaccines have taken just one of those genes – the one that directs the production of the now infamous spike protein that appears on the surface of a normal virus – and injects just that one gene into your muscle cells, which then make that one single protein. Your immune system comes along and sees that weird protein and makes antibodies to it. These same antibodies will now recognize the spike protein on the surface of any viral particles that invade your body. We have effectively turned the virus into its own enemy.
The viral RNA that you are getting will decompose over a few days because RNA is not a stable molecule (that, by the way, is why the vaccine needs to be kept frozen) and it will no longer exist in your body. It could only become a permanent part of your genome if it were a DNA molecule instead of an RNA molecule – and even the chances of that happening would be chemically remote. So regardless of how it sounds, this may actually be the safest sort of vaccine to use. In the future it is likely that all vaccines will be made this way.
Then, of course, there is the issue of who is running this whole vaccine program – the government and the pharmaceutical industry. These are the guys who brought you opioid addiction, death by Vioxx, soaring drug prices, the worst health care system in the developed world, regulations where you don't need them and none where you do – am I really going to trust this cast of so-called "inept villains," as some believe, to dictate my personal health choices? Do we know for sure that the claims of efficacy are real or just made up to sell some worthless procedure? It would not be the first time. (I would not, on the other hand, worry about Bill Gates having a chip inserted into you along with the vaccine – if you use any social media, navigational tools, or purchase anything online, then Bill Gates already knows more about you than he will get from any injectable chip. So that train has left the station.)
The main upside to vaccines is that because they use your already existing defense system, they are surprisingly safe.
The Vaccine Wager
All this and a few lesser issues are worth a pause for sure. But we must also look on the positive side of the ledger. Why trust science? Modern medicine and the science behind it has eliminated or dramatically lessened such scourges as smallpox, polio, cholera, chicken pox, measles, rabies and dozens of other killer pathogens that had previously wiped out enormous numbers of people, in some cases significant parts of entire generations. Don't we depend on science for much of the comfort and safety of our everyday lives? Isn't science the way we heat our homes, drive to work, fly around the world, have dependable food? Yes, there is the bomb – but there is also anesthesia.
When it comes to viruses, the only tool we have to fight them is vaccination. The only tool. Antibiotics are for bacteria, a completely different sort of creature. Sanitation beyond personal hand washing is ineffective. Vaccines trick the immune system into recognizing the virus earlier than it would otherwise and protect normal cells from invasion by the virus. Tricking the immune system is understandably problematic for people who believe that their body knows best if it's just kept healthy. This virus, as we have seen from the array of infected people that includes apparently healthy folks, unfortunately does not subscribe to that belief.
By a similar sort of reasoning, some people make the plausible error of calculating that the vaccine is 95% effective but the survival rate is 99%, so why not just let my natural resistance take care of this? Indeed, that might not be unreasonable thinking if we were talking about the common cold, but this virus has shown itself to be a tricky character and we are not yet able to predict who gets a serious case and who a mild one. With those sorts of stakes, you shouldn't wager on either of those numbers because they have nothing to do with you as an individual. Like flipping a coin, there is only a 1% chance of it coming up heads 6 times in a row. But if it has come up heads 5 times in a row the probability of it coming up heads on the next flip is … still 50/50.
An even larger unknown is whether there may be long-term effects associated with SARS-Cov-2, as is the case for many viruses. The 1918 influenza virus has been linked to a subsequent 2-3 fold increase in Parkinson's disease by a mechanism we still don't understand. The virus that gives children chicken pox will hide out in a person's body for 40 years or more and then emerge as a painful, sometimes debilitating, case of shingles. The 99% survivability rate of this virus is meaningless if 20 years from now it causes some devastating pulmonary or brain disease.
The main upside to vaccines is that because they use your already existing defense system, they are surprisingly safe. Safer than antibiotics which have numerous side effects because they are not part of our normal make up and are cell killers – mostly bacterial cells, but they are not so perfectly targeted that they don't leave some collateral damage in their wake. All drugs and treatments have side effects, but vaccines in general have the fewest. This vaccine in particular has undergone many more than the usual safety measures - multiple independent review boards, massive press and public attention, governmental and non-governmental oversight, the most diverse trial cohorts ever assembled. Nothing here was rushed, no shortcuts were taken.
So here's the vaccine wager. Vaccines are the safest medical procedure we have. They are also among the most effective, but that's curiously not important for the bet. My claim about their safety is because vaccines are in a special class of medical tools. They are the only medical procedure or drug that is given to healthy people. Every other treatment we use medically is aimed at some existing pathology - from a cold to cancer.
Vaccines therefore have to reach a higher standard of safety than any other medical treatment. You can't take healthy people and make them sick. Vaccines have fewer side effects than virtually any other drug you wouldn't even think twice about taking – aspirin, for instance, which can cause internal bleeding, gastric ulcers, stroke. But since you are sick when you take those drugs you are willing to make the bet that the benefits will outweigh the possible side effects.
With vaccines the wager is much simpler – it is indeed more like Pascal's original wager. It may or may not be highly effective (some vaccines are only 60% effective) but they are so safe that taking them poses little risk, whereas not taking them subjects you (and others) to considerable risk, i.e., getting the virus. Like believing or not in an afterlife, the smart money is with Pascal, who I think would have reasoned himself right to the head of the vaccination line.
New tech for prison reform spreads to 11 states
The U.S. has the highest incarceration rate in the world, costing $182 billion per year, partly because its antiquated data systems often fail to identify people who should be released. A tech nonprofit is trying to change that.
A new non-profit called Recidiviz is using data technology to reduce the size of the U.S. criminal justice system. The bi-coastal company (SF and NYC) is currently working with 11 states to improve their systems and, so far, has helped remove nearly 69,000 people — ones left floundering in jail or on parole when they should have been released.
“The root cause is fragmentation,” says Clementine Jacoby, 31, a software engineer who worked at Google before co-founding Recidiviz in 2019. In the 1970s and 80s, the U.S. built a series of disconnected data systems, and this patchwork is still being used by criminal justice authorities today. It requires parole officers to manually calculate release dates, leading to errors in many cases. “[They] have done everything they need to do to earn their release, but they're still stuck in the system,” Jacoby says.
Recidiviz has built a platform that connects the different databases, with the goal of identifying people who are already qualified for release but remain behind bars or on supervision. “Think of Recidiviz like Google Maps,” says Jacoby, who worked on Maps when she was at the tech giant. Google Maps takes in data from different sources – satellite images, street maps, local business data — and organizes it into one easy view. “Recidiviz does something similar with criminal justice data,” Jacoby explains, “making it easy to identify people eligible to come home or to move to less intensive levels of supervision.”
People like Jacoby’s uncle. His experience with incarceration is what inspired her passion for criminal justice reform in the first place.
The problems are vast
The U.S. has the highest incarceration rate in the world — 2 million people according to the watchdog group, Prison Policy Initiative — at a cost of $182 billion a year. The numbers could be a lot lower if not for an array of problems including inaccurate sentencing calculations, flawed algorithms and parole violations laws.
Sentencing miscalculations
To determine eligibility for release, the current system requires corrections officers to check 21 different requirements spread across five different databases for each of the 90 to 100 people under their supervision. These manual calculations are time prohibitive, says Jacoby, and fall victim to human error.
In addition, Recidiviz found that policies aimed at helping to reduce the prison population don’t always work correctly. A key example is time off for good behavior laws that allow inmates to earn one day off for every 30 days of good behavior. Some states' data systems are built to calculate time off as one day per month of good behavior, rather than per day. Over the course of a decade-long sentence, Jacoby says these miscalculations can lead to a huge discrepancy in the calculated release data and the actual release date.
Algorithms
Commercial algorithm-based software systems for risk assessment continue to be widely used in the criminal justice system, even though a 2018 study published in Science Advances exposed their limitations. After the study went viral, it took three years for the Justice Department to issue a report on their own flawed algorithms used to reduce the federal prison population as part of the 2018 First Step Act. The program, it was determined, overestimated the risk of putting inmates of color into early-release programs.
Despite its name, Recidiviz does not build these types of algorithms for predicting recidivism, or whether someone will commit another crime after being released from prison. Rather, Jacoby says the company’s "descriptive analytics” approach is specifically intended to weed out incarceration inequalities and avoid algorithmic pitfalls.
Parole violation laws
Research shows that 350,000 people a year — about a quarter of the total prison population — are sent back not because they’ve committed another crime, but because they’ve broken a specific rule of their probation. “Things that wouldn't send you or I to prison, but would send someone on parole,” such as crossing county lines or being in the presence of alcohol when they shouldn’t be, are inflating the prison population, says Jacoby.
It’s personal for the co-founder and CEO
“I grew up with an uncle who went into the prison system,” Jacoby says. At 19, he was sentenced to ten years in prison for a non-violent crime. A few months after being released from jail, he was sent back for a non-violent parole violation.
“For my family, the fact that one in four prison admissions are driven not by a crime but by someone who's broken a rule on probation and parole was really profound because that happened to my uncle,” Jacoby says. The experience led her to begin studying criminal justice in high school, then college. She continued her dive into how the criminal justice system works as part of her Passion Project while at Google, a program that allows employees to spend 20 percent of their time on pro-bono work. Two colleagues whose family members had also been stuck in the system joined her.
As part of the project, Jacoby interviewed hundreds of people involved in the criminal justice system. “Those on the right, those on the left, agreed that bad data was slowing down reform,” she says. Their research brought them to North Dakota where they began to understand the root of the problem. The corrections department is making “huge, consequential decisions every day [without] … the data,” Jacoby says. In a new video by Recidiviz not yet released, Jacoby recounts her exchange with the state’s director of corrections who told her, “‘It’s not that we have the data and we just don’t know how to make it public; we don’t have the information you think we have.'"
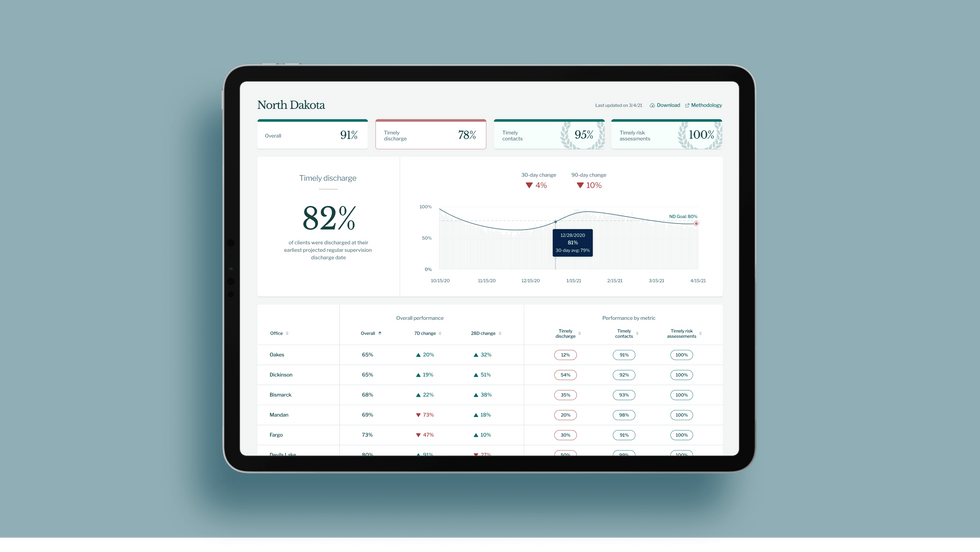
A mock-up (with fake data) of the types of dashboards and insights that Recidiviz provides to state governments.
Recidiviz
As a software engineer, Jacoby says the comment made no sense to her — until she witnessed it first-hand. “We spent a lot of time driving around in cars with corrections directors and parole officers watching them use these incredibly taxing, frankly terrible, old data systems,” Jacoby says.
As they weeded through thousands of files — some computerized, some on paper — they unearthed the consequences of bad data: Hundreds of people in prison well past their release date and thousands more whose release from parole was delayed because of minor paperwork issues. They found individuals stuck in parole because they hadn’t checked one last item off their eligibility list — like simply failing to provide their parole officer with a paystub. And, even when parolees advocated for themselves, the archaic system made it difficult for their parole officers to confirm their eligibility, so they remained in the system. Jacoby and her team also unpacked specific policies that drive racial disparities — such as fines and fees.
The Solution
It’s more than a trivial technical challenge to bring the incomplete, fragmented data onto a 21st century data platform. It takes months for Recidiviz to sift through a state’s information systems to connect databases “with the goal of tracking a person all the way through their journey and find out what’s working for 18- to 25-year-old men, what’s working for new mothers,” explains Jacoby in the video.
TED Talk: How bad data traps people in the U.S. justice system

TED Fellow Clementine Jacoby's TED Talk went live on Jan. 13. It describes how we can fix bad data in the criminal justice system, "bringing thousands of people home, reducing costs and improving public safety along the way."
Clementine Jacoby • TED2022
Ojmarrh Mitchell, an associate professor in the School of Criminology and Criminal Justice at Arizona State University, who is not involved with the company, says what Recidiviz is doing is “remarkable.” His perspective goes beyond academic analysis. In his pre-academic years, Mitchell was a probation officer, working within the framework of the “well known, but invisible” information sharing issues that plague criminal justice departments. The flexibility of Recidiviz’s approach is what makes it especially innovative, he says. “They identify the specific gaps in each jurisdiction and tailor a solution for that jurisdiction.”
On the downside, the process used by Recidiviz is “a bit opaque,” Mitchell says, with few details available on how Recidiviz designs its tools and tracks outcomes. By sharing more information about how its actions lead to progress in a given jurisdiction, Recidiviz could help reformers in other places figure out which programs have the best potential to work well.
The eleven states in which Recidiviz is working include California, Colorado, Maine, Michigan, Missouri, Pennsylvania and Tennessee. And a pilot program launched last year in Idaho, if scaled nationally, with could reduce the number of people in the criminal justice system by a quarter of a million people, Jacoby says. As part of the pilot, rather than relying on manual calculations, Recidiviz is equipping leaders and the probation officers with actionable information with a few clicks of an app that Recidiviz built.
Mitchell is disappointed that there’s even the need for Recidiviz. “This is a problem that government agencies have a responsibility to address,” he says. “But they haven’t.” For one company to come along and fill such a large gap is “remarkable.”
How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next
Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”

