Virtual Clinical Trials Are Letting More People of Color Participate in Research

Virtual clinical trials are helping to eradicate logistical barriers to clinical trial participation, though trust is still an issue.
Herman Taylor, director of the cardiovascular research institute at Morehouse college, got in touch with UnitedHealth Group early in the pandemic.
The very people who most require solutions to COVID are those who are least likely to be involved in the search to find them.
A colleague he worked with at Grady Hospital in Atlanta was the guy when it came to studying sickle cell disease, a recessive genetic disorder that causes red blood cells to harden into half-moon shapes, causing cardiovascular problems. Sickle cell disease is more common in African Americans than it is in Caucasians, in part because having just one gene for the disease, called sickle cell trait, is protective against malaria, which is endemic to much of Africa. Roughly one in 12 African Americans carry sickle cell trait, and Taylor's colleague wondered if this could be one factor affecting differential outcomes for COVID-19.
UnitedHealth Group granted Taylor and his colleague the money to study sickle cell trait in COVID, and then, as they continued working together, they began to ask Taylor his opinion on other topics. As an insurance company, United had realized early in the pandemic that it was sitting on a goldmine of patient data—some 120 million patients' worth—that it could sift through to look for potential COVID treatments.
Their researchers thought they had found one: In a small subset of 14,000 people who'd contracted COVID, there was a group whose bills were paid by Medicare (which the researchers took as a proxy for older age). The people in this group who were taking ACE inhibitors, blood vessel dilators often used to treat high blood pressure, were 40 percent less likely to be hospitalized than those who were not taking the drug.
The connection between ACE inhibitors and COVID hospitalizations was a correlation, a statistical association. To determine whether the drugs had any real effect on COVID outcomes, United would have to perform another, more rigorous study. They would have to assign some people to receive small doses of ACE inhibitors, and others to receive placebos, and measure the outcomes under each condition. They planned to do this virtually, allowing study participants to sign up and be screened online, and sending drugs, thermometers, and tests through the mail. There were two reasons to do it this way: First, the U.S. Food and Drug Administration had been advising medical researchers to embrace new strategies in clinical trials as a way to protect participants during the pandemic.
The second reason was why they asked Herman Taylor to co-supervise it: Clinical trials have long had a diversity problem. And going virtual is a potential solution.
Since the beginning of the pandemic, COVID-19 has infected people of color at a rate of three times that of Caucasians (killing Black people at a rate 2.5 times as high, and Hispanic and American Indian or Alaska Native people at a rate 1.3 times as high). A number of explanations have been put forth to explain this disproportionate toll. Among them: higher levels of poverty, essential jobs that increase exposure, and lower quality or inadequate access to medical care.
Unfortunately, these same factors also affect who participates in research. People of color may be less likely to have doctors recommend studies to them. They may not have the time or the resources to hang out in a waiting room for hours. They may not live near large research institutions that conduct trials. The result is that new treatments, even for diseases that affect Latin, Native American, or African American populations in greater proportions, are studied mostly in white volunteers. The very people who most require solutions to COVID are those who are least likely to be involved in the search to find them.
Virtual trials can alleviate a number of these problems. Not only can people find and request to participate in these types of trials through their phones or computers, virtual trials also cover more costs, include a larger geographical range, and have inherently flexible hours.
"[In a traditional study] you have to go to a doctor's office to enroll and drive a couple of hours and pay $20 for parking and pay $15 for a sandwich in the hospital cafeteria and arrange for daycare for your kids and take time off of work," says Dr. Jonathan Cotliar, chief medical officer of Science37, a platform that investigators can hire to host and organize their trials virtually. "That's a lot just for one visit, much less over the course of a six to 12-month study."
Cotliar's data suggests that virtual trials' enhanced access seriously affects the racial makeup of a given study's participant pool. Sixty percent of patients enrolled in Science37 trials are non-Caucasian, which is, Cotliar says, "staggering compared to what you find in traditional site-based research."
But access is not the only barrier to including more people of color in clinical trials. There is also trust. When agreeing to sign up for research, undocumented immigrants may worry about finding themselves in legal trouble or without any medical support should something go wrong. In a country with a history of experimenting on African Americans without their consent, black people may not trust institutions not to use them as guinea pigs.
"A lot of people report being somewhat disregarded or disrespected once entering the healthcare system," Taylor says. "You take it all together, then people wonder, well, okay, this is how the system tends to regard me, yet now here come these people doing research, and they're all about getting me into their studies." Not so surprising that a lot of people may respond with a resounding "No thanks."
United's ACE inhibitor trial was notable for addressing both of these challenges. In addition to covering costs and allowing study subjects to participate from their own homes, it was being co-sponsored by a professor at Morehouse, one of the country's historic black colleges and universities (often abbreviated HBCUs). United was recruiting heavily in Atlanta, whose population is 52 percent African American. The study promised a thoughtful introduction to a more egalitarian future of medical research.
There's just one problem: It isn't going to happen.
This month, in preparation for the study, United reanalyzed their ACE inhibitor data with all the new patients who'd contracted COVID in the months since their first analysis. Their original data set had been concentrated in the Northeast, mostly New York City, where the earliest outbreak took place. In the 12 weeks it had taken them to set up the virtual followup study, epicenters had shifted. United's second, more geographically comprehensive sample had ten times the number of people in it. And in that sample, the signal simply disappeared.
"I was shocked, but that's the reality," says Deneen Vojta, executive vice president of enterprise research and development for UnitedHealth Group. "You make decisions based on the data, but when you get more data, more information, you might make a different decision. The answer is the answer."
There was no point in running a virtual ACE inhibitor study if a larger, more representative sample of people indicated the drug was unlikely to help anyone. Still, the model United had established to run the trial remains viable. Even as she scrapped the ACE inhibitor study, Vojta hoped not just to continue United's relationship with Dr. Taylor and Morehouse, but to formalize it. Virtual platforms are still an important part of their forthcoming trials.
If people don't believe a vaccine has been created with them in mind, then they won't take it, and it won't matter whether it exists or not.
United is not alone in this approach. As phase three trials for vaccines against SARS CoV-2 get underway, big pharma companies have been publicly articulating their own strategies for including more people of color in clinical trials, and many of these include virtual elements. Janelle Sabo, global head of clinical innovation, systems and clinical supply chain at Eli Lilly, told me that the company is employing home health and telemedicine, direct-to-patient shipping and delivery, and recruitment using social media and geolocation to expand access to more diverse populations.
Dr. Macaya Douoguih, Head of Clinical Development and Medical Affairs for Janssen Vaccines under Johnson & Johnson, spoke to Congress about this issue during a July hearing before the House Energy and Commerce Oversight and Investigations Subcommittee. She said that the company planned to institute a "focused digital and community outreach plan to provide resources and opportunities to encourage participation in our clinical trials," and had partnered with Johns Hopkins Bloomberg School of Public Health "to understand how the COVID-19 crisis is affecting different communities in the United States."
But while some of these plans are well thought-out, others are concerningly nebulous, featuring big pronouncements but fewer tangible strategies. In that same July hearing, Massachusetts representative Joe Kennedy III (D) sounded like a frustrated teacher when admonishing four of the five leads of the present pharma companies (AstraZeneca, Johnson & Johnson, Merck, Moderna, and Pfizer) for not explaining exactly how they'd ensure diversity both in the study of their vaccines, and in their eventual distribution.
This matters: The uptake of the flu vaccine is 10 percentage points lower in both the African American and Hispanic communities than it is in Caucasians. A Pew research study conducted early in the pandemic found that just 54 percent of Black adults said they "would definitely or probably get a coronavirus vaccine," compared to 74 percent of Whites and Hispanics.
"As a good friend of mine, Dr. [James] Hildreth, president at Meharry, another HBC medical school, likes to say: 'A vaccine is great, but it is the vaccination that saves people,'" Taylor says. If people don't believe a vaccine has been created with them in mind, then they won't take it, and it won't matter whether it exists or not.
In this respect, virtual platforms remain an important first step, at least in expanding admittance. In June, United Health opened up a trial to their entire workforce for a computer game that could treat ADHD. In less than two months, 1,743 people had signed up for it, from all different socioeconomic groups, from all over the country. It was inching closer to the kind of number you need for a phase three vaccine trial, which can require tens of thousands of people. Back when they'd been planning the ACE inhibitor study, United had wanted 9,600 people to agree to participate.
Now, with the help of virtual enrollment, they hope they can pull off similarly high numbers for the COVID vaccine trial they will be running for an as-yet-unnamed pharmaceutical partner. It stands to open in September.
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
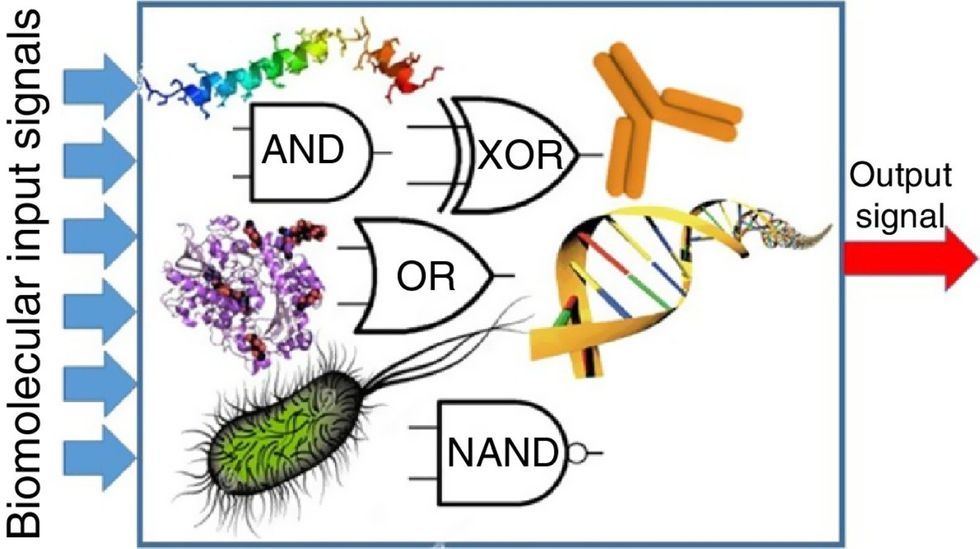
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.