Virtual Reality is Making Medical Care for Kids Less Scary and Painful
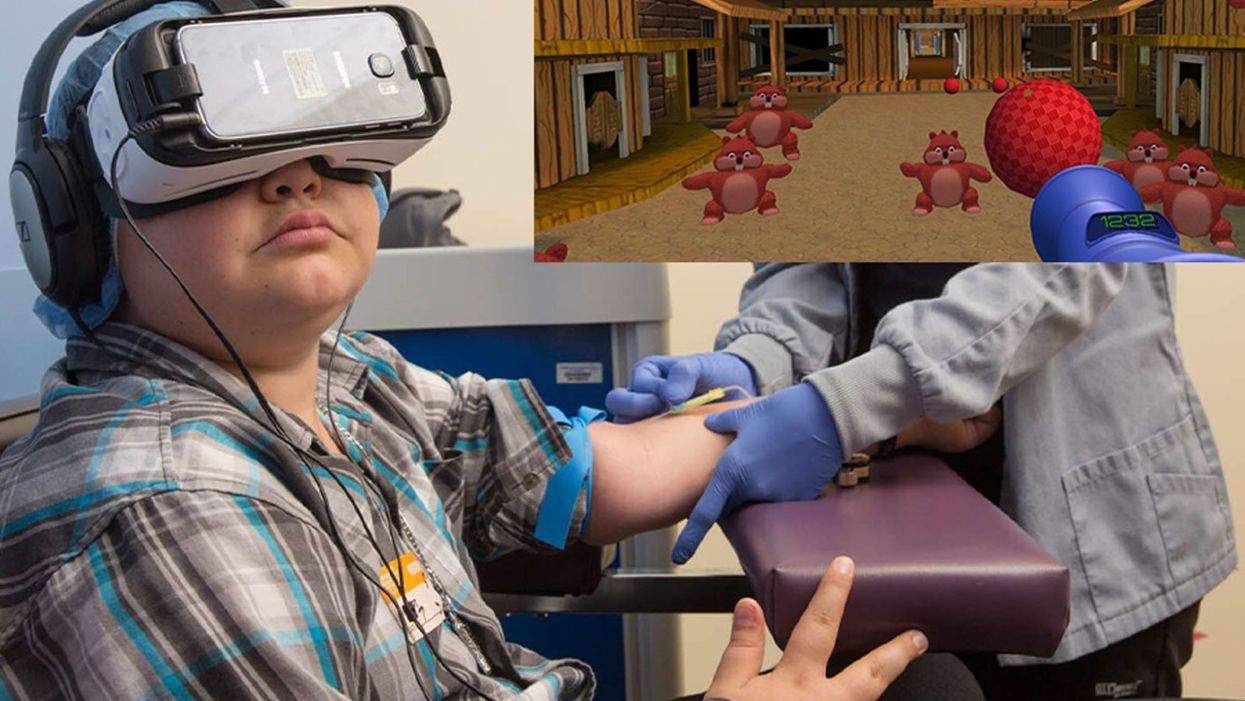
A patient at Children's Hospital Los Angeles (Courtesy of Children's Hospital Los Angeles) plus Bear Blast, developed by AppliedVR.
A blood draw is not normally a fun experience, but these days, virtual reality technology is changing that.
Instead of watching a needle go into his arm, a child wearing a VR headset at Children's Hospital Los Angeles can play a game throwing balls at cartoon bears. In Seattle, at the University of Washington, a burn patient can immerse herself in a soothing snow scene. And at the University of Miami Hospital, a five-minute skin biopsy can become an exciting ride at an amusement park.
VR is transforming once-frightening medical encounters for kids, from blood draws to biopsies to pre-surgical prep, into tolerable ones.
It's literally a game changer, says pediatric neurosurgeon Kurtis Auguste, who uses the tool to help explain pending operations to his young patients and their families. The virtual reality 3-D portrait of their brain is recreated from an MRI, originally to help plan the surgery. The image of normally bland tissue is painted with false colors to better see the boundaries and anomalies of each component. It can be rotated, viewed from every possible angle, zoomed in and out; incisions can be made and likely results anticipated. Auguste has extended its use to patients and families.
"The moment you put these headsets on the kids, we immediately have a link, because honestly, this is how they communicate with each other," says Auguste. "We're all sitting around the table playing games. It's really bridged the distance between me, the pediatric specialist, and my patients" at the Benioff Children's Hospital Oakland, now affiliated with the University of California San Francisco School of Medicine.
The VR experience engages people where they are, immersing them in the environment rather than lecturing them. And it seems to work in all environments, across age and cultural differences, leading to a better grasp of what will be undertaken. That understanding is crucial to meaningful informed consent for surgery. It is particularly relevant for safety-net hospitals, which includes most children's hospitals, because often members of the families were born elsewhere and may have limited understanding of English, not to mention advanced medicine.
Targeting pain
"We're trying to target ways that we can decrease pain, anxiety, fear – what people usually experience as a function of a needle," says Jeffrey Gold, a pioneer in adapting VR at Children's Hospital Los Angeles. He ran the pain clinic there and in 2004 initially focused on phlebotomy, simple blood draws. Many of their kids require frequent blood draws to monitor serious chronic conditions such as diabetes, HIV infection, sickle cell disease, and other conditions that affect the heart, liver, kidneys and other organs.
The scientific explanation of how VR works for pain relief draws upon two basic principles of brain function. The first is "top down inhibition," Gold explains. "We all have the inherent capacity to turn down signals once we determine that signal is no longer harmful, dangerous, hurtful, etc. That's how our brain operates on purpose. It's not just a distraction, it's actually your brain stopping the pain signal at the spinal cord before it can fire all the way up to the frontal lobe."
Second is the analgesic effect from endorphins. "If you're in a gaming environment, and you're having fun and you're laughing and giggling, you are actually releasing endorphins...a neurochemical reaction at the synaptic level of the brain," he says.
Part of what makes VR effective is "what's called a cognitive load, where you have to actually learn something and do something," says Gold. He has worked with developers on a game call Bear Blast, which has proven to be effective in a clinical trial for mitigating pain. But he emphasizes, it is not a one-size-fits all; the programs and patients need to be evaluated to understand what works best for each case.
Gold was a bit surprised to find that VR "actually facilitates quicker blood draws," because the staff doesn't have to manage the kids' anxiety, so "they require fewer needle sticks." The kids, parents, and staff were all having a good time, "and that's a big win when everybody is benefiting." About two years ago the hospital made VR an option that patients can request in the phlebotomy lab, and about half of kids age 4 and older choose to do so.
The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery.
VR reduces or eliminates the need to use sedation or anesthesia, which carries a small but real risk of an adverse reaction. And important to parents, it eliminates the recovery time from using sedation, which shortens the visit and time missed from school and work.
A more intriguing question is whether reducing fear and anxiety in early-life experiences with the healthcare system through activities like VR will have a long-term affect on kids' attitudes toward medicine as they grow older. "If you're a screaming meemie when you come get your blood draw when you're five or seven, you're still that anxious adolescent or adult who is all quivering and sweating and avoiding healthcare," Gold says. "That's a longitudinal health outcome I'd love to get my hands on in 10-15 years from now."
Broader applications
Dermatologist Hadar Lev-Tov read about the use of VR to treat pain and decided to try it in his practice at the University of Miami Hospital. He thought, "OK, this is low risk, it's easy to do. So we got some equipment and got it done." It was so affordable he paid for it out of his own pocket, rather than wait to go through administrative channels. The results were so interesting that he decided to publish it as a series of case studies with a wide variety of patients and types of procedures.
Some of them, such as freezing off warts, are not particularly painful. "But there can be a lot of anxiety, especially for kids, which can be worse than pain and can disrupt the procedure." It can trigger a non-rational, primal fight or flight response in the limbic region of the brain.
Adults understand the need for a biopsy of a skin growth and tolerate what might be a momentary flick of pain. "But for a kid you think twice about a biopsy, both because it's a hassle and because it could be a traumatic event for a child," says Lev-Tov. VR has helped to allay such fears and improve medical care.
Integrating VR into practice has been relatively easy, primarily focusing on simple training for staff and ensuring that standard infection control practices are used in handling equipment that is used by different patients. More mundane issues are ensuring that the play back and wi-fi equipment are functioning properly. He has had a few complaints from kids when the procedure is competed and the VR is turned off prematurely, which is why he favors programs like a roller coaster ride that lasts about five minutes, ample time to take a biopsy or two.
The future is today
The pediatric neurosurgeon Auguste is collaborating with colleagues at Oakland Children's to expand use of VR into different areas of care. Cancer specialists often use a port, a bubble installed under the skin in the chest of the child, to administer chemotherapy. But the young patient's curiosity often draws their attention downward to the port and their chin can potentially contaminate or obstruct it, interfering with the procedure. So the team developed a VR game involving birds that requires players to move their heads upward, away from the port, improving administration of the drugs and reducing the risk of infection.
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor.
Other games are being developed for rehabilitation that require the use of specific nerve and muscle combinations. The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery, Auguste explains. "We can monitor their progress by the score on the game, and if it plateaus, maybe switch to another game."
Another project is trying to ease the anxiety and confusion of the patient and family experience within the hospital itself. Hospital staff are creating a personalized VR introductory walking tour that leads from the parking garage through the maze of structures and corridors in the hospital complex to Dr. Auguste's office, phlebotomy, the MRI site, and other locations they might visit. The goal is to make them familiar with key landmarks before they even set foot in the facility. "So when they come the day of the visit they have already taken that exact same path, hopefully more than once."
"They don't miss their MRI appointment and therefore they don't miss their clinical appointment with me," says Auguste. It reduces patient anxiety about the encounter and from the hospital's perspective, it will reduce costs of missed and rescheduled visits simply because patients did not go to the right place at the right time.
The VR visit will be emailed to patients ahead of time and they can watch it on a smartphone installed in a disposable cardboard viewer. Oakland Children's hopes to have the system in place by early next year. Auguste says their goal in using VR, like other health care providers across the country, is "to streamline the entire patient experience."
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor. That would be a boon to kids, parents, and the health of America.
Regenerative medicine has come a long way, baby
After a cloned baby sheep, what started as one of the most controversial areas in medicine is now promising to transform it.
The field of regenerative medicine had a shaky start. In 2002, when news spread about the first cloned animal, Dolly the sheep, a raucous debate ensued. Scary headlines and organized opposition groups put pressure on government leaders, who responded by tightening restrictions on this type of research.
Fast forward to today, and regenerative medicine, which focuses on making unhealthy tissues and organs healthy again, is rewriting the code to healing many disorders, though it’s still young enough to be considered nascent. What started as one of the most controversial areas in medicine is now promising to transform it.
Progress in the lab has addressed previous concerns. Back in the early 2000s, some of the most fervent controversy centered around somatic cell nuclear transfer (SCNT), the process used by scientists to produce Dolly. There was fear that this technique could be used in humans, with possibly adverse effects, considering the many medical problems of the animals who had been cloned.
But today, scientists have discovered better approaches with fewer risks. Pioneers in the field are embracing new possibilities for cellular reprogramming, 3D organ printing, AI collaboration, and even growing organs in space. It could bring a new era of personalized medicine for longer, healthier lives - while potentially sparking new controversies.
Engineering tissues from amniotic fluids
Work in regenerative medicine seeks to reverse damage to organs and tissues by culling, modifying and replacing cells in the human body. Scientists in this field reach deep into the mechanisms of diseases and the breakdowns of cells, the little workhorses that perform all life-giving processes. If cells can’t do their jobs, they take whole organs and systems down with them. Regenerative medicine seeks to harness the power of healthy cells derived from stem cells to do the work that can literally restore patients to a state of health—by giving them healthy, functioning tissues and organs.
Modern-day regenerative medicine takes its origin from the 1998 isolation of human embryonic stem cells, first achieved by John Gearhart at Johns Hopkins University. Gearhart isolated the pluripotent cells that can differentiate into virtually every kind of cell in the human body. There was a raging controversy about the use of these cells in research because at that time they came exclusively from early-stage embryos or fetal tissue.
Back then, the highly controversial SCNT cells were the only way to produce genetically matched stem cells to treat patients. Since then, the picture has changed radically because other sources of highly versatile stem cells have been developed. Today, scientists can derive stem cells from amniotic fluid or reprogram patients’ skin cells back to an immature state, so they can differentiate into whatever types of cells the patient needs.
In the context of medical history, the field of regenerative medicine is progressing at a dizzying speed. But for those living with aggressive or chronic illnesses, it can seem that the wheels of medical progress grind slowly.
The ethical debate has been dialed back and, in the last few decades, the field has produced important innovations, spurring the development of whole new FDA processes and categories, says Anthony Atala, a bioengineer and director of the Wake Forest Institute for Regenerative Medicine. Atala and a large team of researchers have pioneered many of the first applications of 3D printed tissues and organs using cells developed from patients or those obtained from amniotic fluid or placentas.
His lab, considered to be the largest devoted to translational regenerative medicine, is currently working with 40 different engineered human tissues. Sixteen of them have been transplanted into patients. That includes skin, bladders, urethras, muscles, kidneys and vaginal organs, to name just a few.
These achievements are made possible by converging disciplines and technologies, such as cell therapies, bioengineering, gene editing, nanotechnology and 3D printing, to create living tissues and organs for human transplants. Atala is currently overseeing clinical trials to test the safety of tissues and organs engineered in the Wake Forest lab, a significant step toward FDA approval.
In the context of medical history, the field of regenerative medicine is progressing at a dizzying speed. But for those living with aggressive or chronic illnesses, it can seem that the wheels of medical progress grind slowly.
“It’s never fast enough,” Atala says. “We want to get new treatments into the clinic faster, but the reality is that you have to dot all your i’s and cross all your t’s—and rightly so, for the sake of patient safety. People want predictions, but you can never predict how much work it will take to go from conceptualization to utilization.”
As a surgeon, he also treats patients and is able to follow transplant recipients. “At the end of the day, the goal is to get these technologies into patients, and working with the patients is a very rewarding experience,” he says. Will the 3D printed organs ever outrun the shortage of donated organs? “That’s the hope,” Atala says, “but this technology won’t eliminate the need for them in our lifetime.”
New methods are out of this world
Jeanne Loring, another pioneer in the field and director of the Center for Regenerative Medicine at Scripps Research Institute in San Diego, says that investment in regenerative medicine is not only paying off, but is leading to truly personalized medicine, one of the holy grails of modern science.
This is because a patient’s own skin cells can be reprogrammed to become replacements for various malfunctioning cells causing incurable diseases, such as diabetes, heart disease, macular degeneration and Parkinson’s. If the cells are obtained from a source other than the patient, they can be rejected by the immune system. This means that patients need lifelong immunosuppression, which isn’t ideal. “With Covid,” says Loring, “I became acutely aware of the dangers of immunosuppression.” Using the patient’s own cells eliminates that problem.
Microgravity conditions make it easier for the cells to form three-dimensional structures, which could more easily lead to the growing of whole organs. In fact, Loring's own cells have been sent to the ISS for study.
Loring has a special interest in neurons, or brain cells that can be developed by manipulating cells found in the skin. She is looking to eventually treat Parkinson’s disease using them. The manipulated cells produce dopamine, the critical hormone or neurotransmitter lacking in the brains of patients. A company she founded plans to start a Phase I clinical trial using cell therapies for Parkinson’s soon, she says.
This is the culmination of many years of basic research on her part, some of it on her own cells. In 2007, Loring had her own cells reprogrammed, so there’s a cell line that carries her DNA. “They’re just like embryonic stem cells, but personal,” she said.
Loring has another special interest—sending immature cells into space to be studied at the International Space Station. There, microgravity conditions make it easier for the cells to form three-dimensional structures, which could more easily lead to the growing of whole organs. In fact, her own cells have been sent to the ISS for study. “My colleagues and I have completed four missions at the space station,” she says. “The last cells came down last August. They were my own cells reprogrammed into pluripotent cells in 2009. No one else can say that,” she adds.
Future controversies and tipping points
Although the original SCNT debate has calmed down, more controversies may arise, Loring thinks.
One of them could concern growing synthetic embryos. The embryos are ultimately derived from embryonic stem cells, and it’s not clear to what stage these embryos can or will be grown in an artificial uterus—another recent invention. The science, so far done only in animals, is still new and has not been widely publicized but, eventually, “People will notice the production of synthetic embryos and growing them in an artificial uterus,” Loring says. It’s likely to incite many of the same reactions as the use of embryonic stem cells.
Bernard Siegel, the founder and director of the Regenerative Medicine Foundation and executive director of the newly formed Healthspan Action Coalition (HSAC), believes that stem cell science is rapidly approaching tipping point and changing all of medical science. (For disclosure, I do consulting work for HSAC). Siegel says that regenerative medicine has become a new pillar of medicine that has recently been fast-tracked by new technology.
Artificial intelligence is speeding up discoveries and the convergence of key disciplines, as demonstrated in Atala’s lab, which is creating complex new medical products that replace the body’s natural parts. Just as importantly, those parts are genetically matched and pose no risk of rejection.
These new technologies must be regulated, which can be a challenge, Siegel notes. “Cell therapies represent a challenge to the existing regulatory structure, including payment, reimbursement and infrastructure issues that 20 years ago, didn’t exist.” Now the FDA and other agencies are faced with this revolution, and they’re just beginning to adapt.
Siegel cited the 2021 FDA Modernization Act as a major step. The Act allows drug developers to use alternatives to animal testing in investigating the safety and efficacy of new compounds, loosening the agency’s requirement for extensive animal testing before a new drug can move into clinical trials. The Act is a recognition of the profound effect that cultured human cells are having on research. Being able to test drugs using actual human cells promises to be far safer and more accurate in predicting how they will act in the human body, and could accelerate drug development.
Siegel, a longtime veteran and founding father of several health advocacy organizations, believes this work helped bring cell therapies to people sooner rather than later. His new focus, through the HSAC, is to leverage regenerative medicine into extending not just the lifespan but the worldwide human healthspan, the period of life lived with health and vigor. “When you look at the HSAC as a tree,” asks Siegel, “what are the roots of that tree? Stem cell science and the huge ecosystem it has created.” The study of human aging is another root to the tree that has potential to lengthen healthspans.
The revolutionary science underlying the extension of the healthspan needs to be available to the whole world, Siegel says. “We need to take all these roots and come up with a way to improve the life of all mankind,” he says. “Everyone should be able to take advantage of this promising new world.”
Joy Milne's unusual sense of smell led Dr. Tilo Kunath, a neurobiologist at the Centre for Regenerative Medicine at the University of Edinburgh, and a host of other scientists, to develop a new diagnostic test for Parkinson's.
Forty years ago, Joy Milne, a nurse from Perth, Scotland, noticed a musky odor coming from her husband, Les. At first, Milne thought the smell was a result of bad hygiene and badgered her husband to take longer showers. But when the smell persisted, Milne learned to live with it, not wanting to hurt her husband's feelings.
Twelve years after she first noticed the "woodsy" smell, Les was diagnosed at the age of 44 with Parkinson's Disease, a neurodegenerative condition characterized by lack of dopamine production and loss of movement. Parkinson's Disease currently affects more than 10 million people worldwide.
Milne spent the next several years believing the strange smell was exclusive to her husband. But to her surprise, at a local support group meeting in 2012, she caught the familiar scent once again, hanging over the group like a cloud. Stunned, Milne started to wonder if the smell was the result of Parkinson's Disease itself.
Milne's discovery led her to Dr. Tilo Kunath, a neurobiologist at the Centre for Regenerative Medicine at the University of Edinburgh. Together, Milne, Kunath, and a host of other scientists would use Milne's unusual sense of smell to develop a new diagnostic test, now in development and poised to revolutionize the treatment of Parkinson's Disease.
"Joy was in the audience during a talk I was giving on my work, which has to do with Parkinson's and stem cell biology," Kunath says. "During the patient engagement portion of the talk, she asked me if Parkinson's had a smell to it." Confused, Kunath said he had never heard of this – but for months after his talk he continued to turn the question over in his mind.
Kunath knew from his research that the skin's microbiome changes during different disease processes, releasing metabolites that can give off odors. In the medical literature, diseases like melanoma and Type 2 diabetes have been known to carry a specific scent – but no such connection had been made with Parkinson's. If people could smell Parkinson's, he thought, then it stood to reason that those metabolites could be isolated, identified, and used to potentially diagnose Parkinson's by their presence alone.
First, Kunath and his colleagues decided to test Milne's sense of smell. "I got in touch with Joy again and we designed a protocol to test her sense of smell without her having to be around patients," says Kunath, which could have affected the validity of the test. In his spare time, Kunath collected t-shirt samples from people diagnosed with Parkinson's and from others without the diagnosis and gave them to Milne to smell. In 100 percent of the samples, Milne was able to detect whether a person had Parkinson's based on smell alone. Amazingly, Milne was even able to detect the "Parkinson's scent" in a shirt from the control group – someone who did not have a Parkinson's diagnosis, but would go on to be diagnosed nine months later.
From the initial study, the team discovered that Parkinson's did have a smell, that Milne – inexplicably – could detect it, and that she could detect it long before diagnosis like she had with her husband, Les. But the experiments revealed other things that the team hadn't been expecting.
"One surprising thing we learned from that experiment was that the odor was always located in the back of the shirt – never in the armpit, where we expected the smell to be," Kunath says. "I had a chance meeting with a dermatologist and he said the smell was due to the patient's sebum, which are greasy secretions that are really dense on your upper back. We have sweat glands, instead of sebum, in our armpits." Patients with Parkinson's are also known to have increased sebum production.
With the knowledge that a patient's sebum was the source of the unusual smell, researchers could go on to investigate exactly what metabolites were in the sebum and in what amounts. Kunath, along with his associate, Dr. Perdita Barran, collected and analyzed sebum samples from 64 participants across the United Kingdom. Once the samples were collected, Barran and others analyzed it using a method called gas chromatography mass spectrometry, or GS-MC, which separated, weighed and helped identify the individual compounds present in each sebum sample.
Barran's team can now correctly identify Parkinson's in nine out of 10 patients – a much quicker and more accurate way to diagnose than what clinicians do now.
"The compounds we've identified in the sebum are not unique to people with Parkinson's, but they are differently expressed," says Barran, a professor of mass spectrometry at the University of Manchester. "So this test we're developing now is not a black-and-white, do-you-have-something kind of test, but rather how much of these compounds do you have compared to other people and other compounds." The team identified over a dozen compounds that were present in the sebum of Parkinson's patients in much larger amounts than the control group.
Using only the GC-MS and a sebum swab test, Barran's team can now correctly identify Parkinson's in nine out of 10 patients – a much quicker and more accurate way to diagnose than what clinicians do now.
"At the moment, a clinical diagnosis is based on the patient's physical symptoms," Barran says, and determining whether a patient has Parkinson's is often a long and drawn-out process of elimination. "Doctors might say that a group of symptoms looks like Parkinson's, but there are other reasons people might have those symptoms, and it might take another year before they're certain," Barran says. "Some of those symptoms are just signs of aging, and other symptoms like tremor are present in recovering alcoholics or people with other kinds of dementia." People under the age of 40 with Parkinson's symptoms, who present with stiff arms, are often misdiagnosed with carpal tunnel syndrome, she adds.
Additionally, by the time physical symptoms are present, Parkinson's patients have already lost a substantial amount of dopamine receptors – about sixty percent -- in the brain's basal ganglia. Getting a diagnosis before physical symptoms appear would mean earlier interventions that could prevent dopamine loss and preserve regular movement, Barran says.
"Early diagnosis is good if it means there's a chance of early intervention," says Barran. "It stops the process of dopamine loss, which means that motor symptoms potentially will not happen, or the onset of symptoms will be substantially delayed." Barran's team is in the processing of streamlining the sebum test so that definitive results will be ready in just two minutes.
"What we're doing right now will be a very inexpensive test, a rapid-screen test, and that will encourage people to self-sample and test at home," says Barran. In addition to diagnosing Parkinson's, she says, this test could also be potentially useful to determine if medications were at a therapeutic dose in people who have the disease, since the odor is strongest in people whose symptoms are least controlled by medication.
"When symptoms are under control, the odor is lower," Barran says. "Potentially this would allow patients and clinicians to see whether their symptoms are being managed properly with medication, or perhaps if they're being overmedicated." Hypothetically, patients could also use the test to determine if interventions like diet and exercise are effective at keeping Parkinson's controlled.
"We hope within the next two to five years we will have a test available."
Barran is now running another clinical trial – one that determines whether they can diagnose at an earlier stage and whether they can identify a difference in sebum samples between different forms of Parkinson's or diseases that have Parkinson's-like symptoms, such as Lewy Body Dementia.
"Within the next one to two years, we hope to be running a trial in the Manchester area for those people who do not have motor symptoms but are at risk for developing dementia due to symptoms like loss of smell and sleep difficulty," Barran had said in 2019. "If we can establish that, we can roll out a test that determines if you have Parkinson's or not with those first pre-motor symptoms, and then at what stage. We hope within the next two to five years we will have a test available."
In a 2022 study, published in the American Chemical Society, researchers used mass spectrometry to analyze sebum from skin swabs for the presence of the specific molecules. They found that some specific molecules are present only in people who have Parkinson’s. Now they hope that the same method can be used in regular diagnostic labs. The test, many years in the making, is inching its way to the clinic.
"We would likely first give this test to people who are at risk due to a genetic predisposition, or who are at risk based on prodomal symptoms, like people who suffer from a REM sleep disorder who have a 50 to 70 percent chance of developing Parkinson's within a ten year period," Barran says. "Those would be people who would benefit from early therapeutic intervention. For the normal population, it isn't beneficial at the moment to know until we have therapeutic interventions that can be useful."
Milne's husband, Les, passed away from complications of Parkinson's Disease in 2015. But thanks to him and the dedication of his wife, Joy, science may have found a way to someday prolong the lives of others with this devastating disease. Sometimes she can smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them, Milne said in an interview with the Guardian. But once the test becomes available in the clinics, it will do the job for her.
[Ed. Note: A older version of this hit article originally ran on September 3, 2019.]

