What to Know about the Fast-Spreading Delta Variant
A highly contagious form of the coronavirus known as the Delta variant is spreading rapidly and becoming increasingly prevalent around the world. First identified in India in December, Delta has now been identified in 111 countries.
In the United States, the variant now accounts for 83% of sequenced COVID-19 cases, said Rochelle Walensky, director of the Centers for Disease Control and Prevention, at a July 20 Senate hearing. In May, Delta was responsible for just 3% of U.S. cases. The World Health Organization projects that Delta will become the dominant variant globally over the coming months.
So, how worried should you be about the Delta variant? We asked experts some common questions about Delta.
What is a variant?
To understand Delta, it's helpful to first understand what a variant is. When a virus infects a person, it gets into your cells and makes a copy of its genome so it can replicate and spread throughout your body.
In the process of making new copies of itself, the virus can make a mistake in its genetic code. Because viruses are replicating all the time, these mistakes — also called mutations — happen pretty often. A new variant emerges when a virus acquires one or more new mutations and starts spreading within a population.
There are thousands of SARS-CoV-2 variants, but most of them don't substantially change the way the virus behaves. The variants that scientists are most interested in are known as variants of concern. These are versions of the virus with mutations that allow the virus to spread more easily, evade vaccines, or cause more severe disease.
"The vast majority of the mutations that have accumulated in SARS-CoV-2 don't change the biology as far as we're concerned," said Jennifer Surtees, a biochemist at the University of Buffalo who's studying the coronavirus. "But there have been a handful of key mutations and combinations of mutations that have led to what we're now calling variants of concern."
One of those variants of concern is Delta, which is now driving many new COVID-19 infections.
Why is the Delta variant so concerning?
"The reason why the Delta variant is concerning is because it's causing an increase in transmission," said Alba Grifoni, an infectious disease researcher at the La Jolla Institute for Immunology. "The virus is spreading faster and people — particularly those who are not vaccinated yet — are more prone to exposure."
The Delta variant has a few key mutations that make it better at attaching to our cells and evading the neutralizing antibodies in our immune system. These mutations have changed the virus enough to make it more than twice as contagious as the original SARS-CoV-2 virus that emerged in Wuhan and about 50% more contagious than the Alpha variant, previously known as B.1.1.7, or the U.K. variant.
These mutations were previously seen in other variants on their own, but it's their combination that makes Delta so much more infectious.
Do vaccines work against the Delta variant?
The good news is, the COVID-19 vaccines made by AstraZeneca, Johnson & Johnson, Moderna, and Pfizer still work against the Delta variant. They remain more than 90% effective at preventing hospitalizations and death due to Delta. While they're slightly less protective against disease symptoms, they're still very effective at preventing severe illness caused by the Delta variant.
"They're not as good as they were against the prior strains, but they're holding up pretty well," said Eric Topol, a physician and director of the Scripps Translational Research Institute, during a July 19 briefing for journalists.
Because Delta is better at evading our immune systems, it's likely causing more breakthrough infections — COVID-19 cases in people who are vaccinated. However, breakthrough infections were expected before the Delta variant became widespread. No vaccine is 100% effective, so breakthrough infections can happen with other vaccines as well. Experts say the COVID-19 vaccines are still working as expected, even if breakthrough infections occur. The majority of these infections are asymptomatic or cause only mild symptoms.
Should vaccinated people worry about the Delta variant?
Vaccines train our immune systems to protect us against infection. They do this by spurring the production of antibodies, which stick around in our bodies to help fight off a particular pathogen in case we ever come into contact with it.
But even if the new Delta variant slips past our neutralizing antibodies, there's another component of our immune system that can help overtake the virus: T cells. Studies are showing that the COVID-19 vaccines also galvanize T cells, which help limit disease severity in people who have been vaccinated.
"While antibodies block the virus and prevent the virus from infecting cells, T cells are able to attack cells that have already been infected," Grifoni said. In other words, T cells can prevent the infection from spreading to more places in the body. A study published July 1 by Grifoni and her colleagues found that T cells were still able to recognize mutated forms of the virus — further evidence that our current vaccines are effective against Delta.
Can fully vaccinated people spread the Delta variant?
Previously, scientists believed it was unlikely for fully vaccinated individuals with asymptomatic infections to spread Covid-19. But the Delta variant causes the virus to make so many more copies of itself inside the body, and high viral loads have been found in the respiratory tracts of people who are fully vaccinated. This suggests that vaccinated people may be able to spread the Delta variant to some degree.
If you have COVID-19 symptoms, even if you're fully vaccinated, you should get tested and isolate from friends and family because you could spread the virus.
What risk does Delta pose to unvaccinated people?
The Delta variant is behind a surge in cases in communities with low vaccination rates, and unvaccinated Americans currently account for 97% of hospitalizations due to COVID-19, according to Walensky. The best thing you can do right now to prevent yourself from getting sick is to get vaccinated.
Gigi Gronvall, an immunologist and senior scholar at the Johns Hopkins Center for Health Security, said in this week's "Making Sense of Science" podcast that it's especially important to get all required doses of the vaccine in order to have the best protection against the Delta variant. "Even if it's been more than the allotted time that you were told to come back and get the second, there's no time like the present," she said.
With more than 3.6 billion COVID-19 doses administered globally, the vaccines have been shown to be incredibly safe. Serious adverse effects are rare, although scientists continue to monitor for them.
Being vaccinated also helps prevent the emergence of new and potentially more dangerous variants. Viruses need to infect people in order to replicate, and variants emerge because the virus continues to infect more people. More infections create more opportunities for the virus to acquire new mutations.
Surtees and others worry about a scenario in which a new variant emerges that's even more transmissible or resistant to vaccines. "This is our window of opportunity to try to get as many people vaccinated as possible and get people protected so that so that the virus doesn't evolve to be even better at infecting people," she said.
Does Delta cause more severe disease?
While hospitalizations and deaths from COVID-19 are increasing again, it's not yet clear whether Delta causes more severe illness than previous strains.
How can we protect unvaccinated children from the Delta variant?
With children 12 and under not yet eligible for the COVID-19 vaccine, kids are especially vulnerable to the Delta variant. One way to protect unvaccinated children is for parents and other close family members to get vaccinated.
It's also a good idea to keep masks handy when going out in public places. Due to risk Delta poses, the American Academy of Pediatrics issued new guidelines July 19 recommending that all staff and students over age 2 wear face masks in school this fall, even if they have been vaccinated.
Parents should also avoid taking their unvaccinated children to crowded, indoor locations and make sure their kids are practicing good hand-washing hygiene. For children younger than 2, limit visits with friends and family members who are unvaccinated or whose vaccination status is unknown and keep up social distancing practices while in public.
While there's no evidence yet that Delta increases disease severity in children, parents should be mindful that in some rare cases, kids can get a severe form of the disease.
"We're seeing more children getting sick and we're seeing some of them get very sick," Surtees said. "Those children can then pass on the virus to other individuals, including people who are immunocompromised or unvaccinated."
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
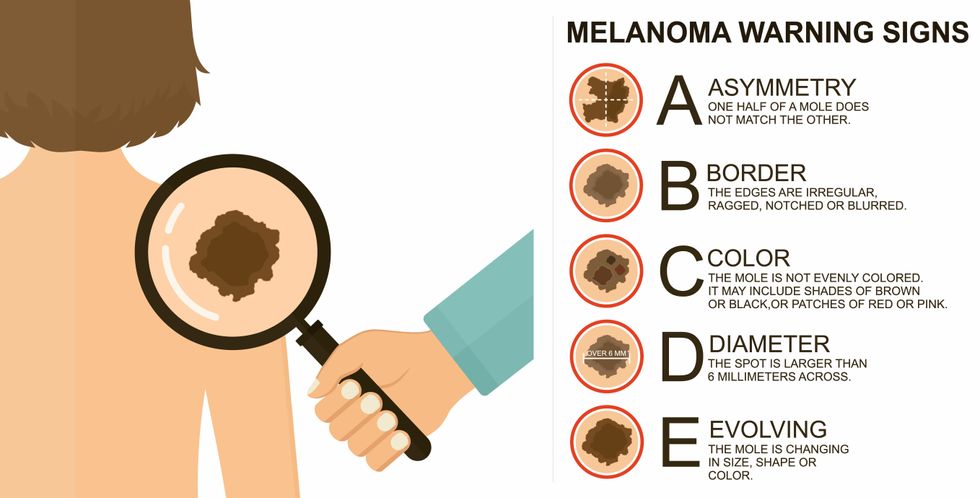
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

