Which Meds are Safe When You’re Pregnant? Science Wants to Find Out

A pregnant woman is uncertain which medicine is safe to take.
Sarah Mancoll was 22 years old when she noticed a bald spot on the back of her head. A dermatologist confirmed that it was alopecia aerata, an autoimmune disorder that causes hair loss.
Of 213 new drugs approved from 2003 to 2012, only five percent included any data from pregnant women.
She successfully treated the condition with corticosteroid shots for nearly 10 years. Then Mancoll and her husband began thinking about starting a family. Would the shots be safe for her while pregnant? For the fetus? What about breastfeeding?
Mancoll consulted her primary care physician, her dermatologist, even a pediatrician. Without clinical data, no one could give her a definitive answer, so she stopped treatment to be "on the safe side." By the time her son was born, she'd lost at least half her hair. She returned to her Washington, D.C., public policy job two months later entirely bald—and without either eyebrows or eyelashes.
After having two more children in quick succession, Mancoll recently resumed the shots but didn't forget her experience. Today, she is an advocate for including more pregnant and lactating women in clinical studies so they can have more information about therapies than she did.
"I live a very privileged life, and I'll do just fine with or without hair, but it's not just about me," Mancoll said. "It's about a huge population of women who are being disenfranchised…They're invisible."
About 4 million women give birth each year in the United States, and many face medical conditions, from hypertension and diabetes to psychiatric disorders. A 2011 study showed that most women reported taking at least one medication while pregnant between 1976 and 2008. But for decades, pregnant and lactating women have been largely excluded from clinical drug studies that rigorously test medications for safety and effectiveness.
An estimated 98 percent of government-approved drug treatments between 2000 and 2010 had insufficient data to determine risk to the fetus, and close to 75 percent had no human pregnancy data at all. All told, of 213 new pharmaceuticals approved from 2003 to 2012, only five percent included any data from pregnant women.
But recent developments suggest that could be changing. Amid widespread concerns about increased maternal mortality rates, women's health advocates, physicians, and researchers are sensing and encouraging a cultural shift toward protecting women through responsible research instead of from research.
"The question is not whether to do research with pregnant women, but how," Anne Drapkin Lyerly, professor and associate director of the Center for Bioethics at the University of North Carolina at Chapel Hill, wrote last year in an op-ed. "These advances are essential. It is well past time—and it is morally imperative—for research to benefit pregnant women."
"In excluding pregnant women from drug trials to protect them from experimentation, we subject them to uncontrolled experimentation."
To that end, the American College of Obstetricians and Gynecologists' Committee on Ethics acknowledged that research trials need to be better designed so they don't "inappropriately constrain the reproductive choices of study participants or unnecessarily exclude pregnant women." A federal task force also called for significantly expanded research and the removal of regulatory barriers that make it difficult for pregnant and lactating women to participate in research.
Several months ago, a government change to a regulation known as the Common Rule took effect, removing pregnant women as a "vulnerable population" in need of special protections -- a designation that had made it more difficult to enroll them in clinical drug studies. And just last week, the U.S. Food and Drug Administration (FDA) issued new draft guidances for industry on when and how to include pregnant and lactating women in clinical trials.
Inclusion is better than the absence of data on their treatment, said Catherine Spong, former chair of the federal task force.
"It's a paradox," said Spong, professor of obstetrics and gynecology and chief of maternal fetal medicine at University of Texas Southwestern Medical Center. "There is a desire to protect women and fetuses from harm, which is translated to a reluctance to include them in research. By excluding them, the evidence for their care is limited."
Jacqueline Wolf, a professor of the history of medicine at Ohio University, agreed.
"In excluding pregnant women from drug trials to protect them from experimentation, we subject them to uncontrolled experimentation," she said. "We give them the medication without doing any research, and that's dangerous."
Women, of course, don't stop getting sick or having chronic medical conditions just because they are pregnant or breastfeeding, and conditions during pregnancy can affect a baby's health later in life. Evidence-based data is important for other reasons, too.
Pregnancy can dramatically change a woman's physiology, affecting how drugs act on her body and how her body acts or reacts to drugs. For instance, pregnant bodies can more quickly clear out medications such as glyburide, used during diabetes in pregnancy to stabilize high blood-sugar levels, which can be toxic to the fetus and harmful to women. That means a regular dose of the drug may not be enough to control blood sugar and prevent poor outcomes.
Pregnant patients also may be reluctant to take needed drugs for underlying conditions (and doctors may be hesitant to prescribe them), which in turn can cause more harm to the woman and fetus than had they been treated. For example, women who have severe asthma attacks while pregnant are at a higher risk of having low-birthweight babies, and pregnant women with uncontrolled diabetes in early pregnancy have more than four times the risk of birth defects.
Current clinical trials involving pregnant women are assessing treatments for obstructive sleep apnea, postpartum hemorrhage, lupus, and diabetes.
For Kate O'Brien, taking medication during her pregnancy was a matter of life and death. A freelance video producer who lives in New Jersey, O'Brien was diagnosed with tuberculosis in 2015 after she became pregnant with her second child, a boy. Even as she signed hospital consent forms, she had no idea if the treatment would harm him.
"It's a really awful experience," said O'Brien, who now is active with We are TB, an advocacy and support network. "All they had to tell me about the medication was just that women have been taking it for a really long time all over the world. That was the best they could do."
More and more doctors, researchers and women's health organizations and advocates are calling that unacceptable.
By indicating that filling current knowledge gaps is "a critical public health need," the FDA is signaling its support for advancing research with pregnant women, said Lyerly, also co-founder of the Second Wave Initiative, which promotes fair representation of the health interests of pregnant women in biomedical research and policies. "It's a very important shift."
Research with pregnant women can be done ethically, Lyerly said, whether by systematically collecting data from those already taking medications or enrolling pregnant women in studies of drugs or vaccines in development.
Current clinical trials involving pregnant women are assessing treatments for obstructive sleep apnea, postpartum hemorrhage, lupus, and diabetes. Notable trials in development target malaria and HIV prevention in pregnancy.
"It clearly is doable to do this research, and test trials are important to provide evidence for treatment," Spong said. "If we don't have that evidence, we aren't making the best educated decisions for women."
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
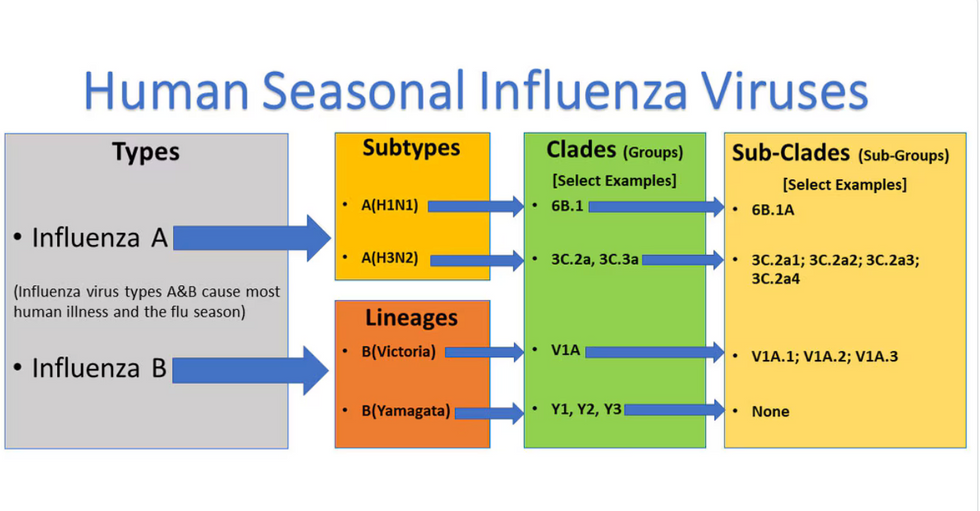
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

