Why Are Autism Rates Steadily Rising?

Stefania Sterling with her son Charlie, who was diagnosed at age 3 with autism.
Stefania Sterling was just 21 when she had her son, Charlie. She was young and healthy, with no genetic issues apparent in either her or her husband's family, so she expected Charlie to be typical.
"It is surprising that the prevalence of a significant disorder like autism has risen so consistently over a relatively brief period."
It wasn't until she went to a Mommy and Me music class when he was one, and she saw all the other one-year-olds walking, that she realized how different her son was. He could barely crawl, didn't speak, and made no eye contact. By the time he was three, he was diagnosed as being on the lower functioning end of the autism spectrum.
She isn't sure why it happened – and researchers, too, are still trying to understand the basis of the complex condition. Studies suggest that genes can act together with influences from the environment to affect development in ways that lead to Autism Spectrum Disorder (ASD). But rates of ASD are rising dramatically, making the need to figure out why it's happening all the more urgent.
The Latest News
Indeed, the CDC's latest autism report, released last week, which uses 2016 data, found that the prevalence of ASD in four-year-old children was one in 64 children, or 15.6 affected children per 1,000. That's more than the 14.1 rate they found in 2014, for the 11 states included in the study. New Jersey, as in years past, was the highest, with 25.3 per 1,000, compared to Missouri, which had just 8.8 per 1,000.
The rate for eight-year-olds had risen as well. Researchers found the ASD prevalence nationwide was 18.5 per 1,000, or one in 54, about 10 percent higher than the 16.8 rate found in 2014. New Jersey, again, was the highest, at one in 32 kids, compared to Colorado, which had the lowest rate, at one in 76 kids. For New Jersey, that's a 175 percent rise from the baseline number taken in 2000, when the state had just one in 101 kids.
"It is surprising that the prevalence of a significant disorder like autism has risen so consistently over a relatively brief period," said Walter Zahorodny, an associate professor of pediatrics at Rutgers New Jersey Medical School, who was involved in collecting the data.
The study echoed the findings of a surprising 2011 study in South Korea that found 1 in every 38 students had ASD. That was the the first comprehensive study of autism prevalence using a total population sample: A team of investigators from the U.S., South Korea, and Canada looked at 55,000 children ages 7 to 12 living in a community in South Korea and found that 2.64 percent of them had some level of autism.
Searching for Answers
Scientists can't put their finger on why rates are rising. Some say it's better diagnosis. That is, it's not that more people have autism. It's that we're better at detecting it. Others attribute it to changes in the diagnostic criteria. Specifically, the May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders-5 -- the standard classification of mental disorders -- removed the communication deficit from the autism definition, which made more children fall under that category. Cynical observers believe physicians and therapists are handing out the diagnosis more freely to allow access to services available only to children with autism, but that are also effective for other children.
Alycia Halladay, chief science officer for the Autism Science Foundation in New York, said she wishes there were just one answer, but there's not. While she believes the rising ASD numbers are due in part to factors like better diagnosis and a change in the definition, she does not believe that accounts for the entire rise in prevalence. As for the high numbers in New Jersey, she said the state has always had a higher prevalence of autism compared to other states. It is also one of the few states that does a good job at recording cases of autism in its educational records, meaning that children in New Jersey are more likely to be counted compared to kids in other states.
"Not every state is as good as New Jersey," she said. "That accounts for some of the difference compared to elsewhere, but we don't know if it's all of the difference in prevalence, or most of it, or what."
"What we do know is that vaccinations do not cause autism."
There is simply no defined proven reason for these increases, said Scott Badesch, outgoing president and CEO of the Autism Society of America.
"There are suggestions that it is based on better diagnosis, but there are also suggestions that the incidence of autism is in fact increasing due to reasons that have yet been determined," he said, adding, "What we do know is that vaccinations do not cause autism."
Zahorodny, the pediatrics professor, believes something is going on beyond better detection or evolving definitions.
"Changes in awareness and shifts in how children are identified or diagnosed are relevant, but they only take you so far in accounting for an increase of this magnitude," he said. "We don't know what is driving the surge in autism recorded by the ADDM Network and others."
He suggested that the increase in prevalence could be due to non-genetic environmental triggers or risk factors we do not yet know about, citing possibilities including parental age, prematurity, low birth rate, multiplicity, breech presentation, or C-section delivery. It may not be one, but rather several factors combined, he said.
"Increases in ASD prevalence have affected the whole population, so the triggers or risks must be very widely dispersed across all strata," he added.
There are studies that find new risk factors for ASD almost on a daily basis, said Idan Menashe, assistant professor in the Department of Health at Ben-Gurion University of the Negev, the fastest growing research university in Israel.
"There are plenty of studies that find new genetic variants (and new genes)," he said. In addition, various prenatal and perinatal risk factors are associated with a risk of ASD. He cited a study his university conducted last year on the relationship between C-section births and ASD, which found that exposure to general anesthesia may explain the association.
Whatever the cause, health practitioners are seeing the consequences in real time.
"People say rates are higher because of the changes in the diagnostic criteria," said Dr. Roseann Capanna-Hodge, a psychologist in Ridgefield, CT. "And they say it's easier for children to get identified. I say that's not the truth and that I've been doing this for 30 years, and that even 10 years ago, I did not see the level of autism that I do see today."
Sure, we're better at detecting autism, she added, but the detection improvements have largely occurred at the low- to mid- level part of the spectrum. The higher rates of autism are occurring at the more severe end, in her experience.
A Polarizing Theory
Among the more controversial risk factors scientists are exploring is the role environmental toxins may play in the development of autism. Some scientists, doctors and mental health experts suspect that toxins like heavy metals, pesticides, chemicals, or pollution may interrupt the way genes are expressed or the way endocrine systems function, manifesting in symptoms of autism. But others firmly resist such claims, at least until more evidence comes forth. To date, studies have been mixed and many have been more associative than causative.
"Today, scientists are still trying to figure out whether there are other environmental changes that can explain this rise, but studies of this question didn't provide any conclusive answer," said Menashe, who also serves as the scientific director of the National Autism Research Center at BGU.
"It's not everything that makes Charlie. He's just like any other kid."
That inconclusiveness has not dissuaded some doctors from taking the perspective that toxins do play a role. "Autism rates are rising because there is a mismatch between our genes and our environment," said Julia Getzelman, a pediatrician in San Francisco. "The majority of our evolution didn't include the kinds of toxic hits we are experiencing. The planet has changed drastically in just the last 75 years –- it has become more and more polluted with tens of thousands of unregulated chemicals being used by industry that are having effects on our most vulnerable."
She cites BPA, an industrial chemical that has been used since the 1960s to make certain plastics and resins. A large body of research, she says, has shown its impact on human health and the endocrine system. BPA binds to our own hormone receptors, so it may negatively impact the thyroid and brain. A study in 2015 was the first to identify a link between BPA and some children with autism, but the relationship was associative, not causative. Meanwhile, the Food and Drug Administration maintains that BPA is safe at the current levels occurring in food, based on its ongoing review of the available scientific evidence.
Michael Mooney, President of St. Louis-based Delta Genesis, a non-profit organization that treats children struggling with neurodevelopmental delays like autism, suspects a strong role for epigenetics, which refers to changes in how genes are expressed as a result of environmental influences, lifestyle behaviors, age, or disease states.
He believes some children are genetically predisposed to the disorder, and some unknown influence or combination of influences pushes them over the edge, triggering epigenetic changes that result in symptoms of autism.
For Stefania Sterling, it doesn't really matter how or why she had an autistic child. That's only one part of Charlie.
"It's not everything that makes Charlie," she said. "He's just like any other kid. He comes with happy moments. He comes with sad moments. Just like my other three kids."
A Drug Straight Out of Science Fiction Has Arrived
Doctor with a syringe on the background of DNA.
Steve, a 60-year-old resident of the DC area who works in manufacturing, was always physically fit. In college, he played lacrosse in Division I, the highest level of intercollegiate athletics in the United States. Later, he stayed active by swimming, biking, and running--up until something strange happened around two years ago.
"It was hard for me to even get upstairs. I wasted away."
Steve, who requested that his last name be withheld to protect his privacy, started to notice weakness first in his toes, then his knees. On a trip to the zoo, he had trouble keeping up. Then some months later, the same thing happened on a family hike. What was supposed to be a four-mile trek up to see a waterfall ended for him at the quarter-mile mark. He turned around and struggled back to the start just as everyone else was returning from the excursion.
Alarmed, he sought out one doctor after the next, but none could diagnose him. The disabling weakness continued to creep up his legs, and by the time he got in to see a top neurologist at Johns Hopkins University last January, he was desperate for help.
"It was hard for me to even get upstairs," he recalls. "I wasted away and had lost about forty-five pounds."
The neurologist, Dr. Michael Polydefkis, finally made the correct diagnosis based on Steve's rapid progression of symptoms, a skin and nerve biopsy, and a genetic test. It turned out that Steve had a rare inherited disease called hereditary transthyretin amyloidosis. Transthyretin is a common blood protein whose normal function is to transport vitamins and hormones in the body. When patients possess certain genetic mutations in the transthyretin gene, the resulting protein can misfold, clump and produce amyloid, an aggregate of proteins, which then interferes with normal function. Many organs are affected in this disease, but most affected are the nervous system, the GI tract, and the heart.

Dr. Michael Polydefkis, Steve's neurologist at Johns Hopkins Bayview Medical Center in Baltimore, MD.
(Courtesy of Dr. Polydefkis)
For the 50,000 patients like Steve around the world, the only treatment historically has been a liver transplant—a major, risky operation. The liver makes most of the transthyretin in a person's body. So if a person who carries a genetic mutation for a disease-causing form of transthyretin has their liver transplanted, the new liver will stop making the mutant protein. A few drugs can slow, but do not stop the disease.
Since it is a genetic condition, a regular "drug" can't tackle the problem.
"For almost all of medicine from the 18th century to today, drugs have been small molecules, typically natural, some invented by humans, that bind to proteins and block their functions," explains Dr. Phillip Zamore, chair of the department of Biomedical Sciences at the University of Massachusetts Medical School. "But with most proteins (including this one), you can't imagine how that would ever happen. Because even if it stuck, there's no reason to think it would change anything. So people threw up their hands and said, 'Unless we can find a protein that is "druggable" in disease X, we can't treat it.'"
To draw a car analogy, treating a disease like Steve's with a small molecule would be like trying to shut down the entire car industry when all you can do is cut the power cord to one machine in one local factory. With few options, patients like Steve have been at a loss, facing continual deterioration and disability.
"It's more obvious how to be specific because we use the genetic code itself to design the drug."
A Radical New Approach
Luckily, Dr. Polydefkis knew of an experimental drug made by a biotech company that Dr. Zamore co-founded called Alnylam Pharmaceuticals. They were doing something completely different: silencing the chemical blueprint for protein, called RNA, rather than targeting the protein itself. In other words, shutting down all the bad factories across the whole car industry at once – without touching the good ones.
"It's more obvious how to be specific," says Dr. Zamore, "because we use the genetic code itself to design the drug."
For Steve's doctor, the new drug, called patisiran, is a game changer.
"It's the dawn of molecular medicine," says Dr. Polydefkis. "It's really a miraculous development. The ability to selectively knock down or reduce the amount of a specific protein is remarkable. I tell patients this is science fiction that is now becoming reality."
A (Very) Short History
The strategy of silencing RNA as a method of guiding drug development began in 1998. Basic research took six years before clinical testing in humans began in 2004. Just a few months ago, in November, the results of the first double-blind, placebo-controlled phase III trials were announced, testing patisiran in patients--and they surpassed expectations.
"The results were remarkably positive," says Dr. Polydefkis. "Every primary and secondary outcome measure target was met. It's the most positive trial I have ever been associated with and that I can remember in recent memory."
FDA approval is expected to come by summer, which will mark the first official sanction of a drug based on RNA inhibition (RNAi). Experts are confident that similar drugs will eventually follow for other diseases, like familial hypercholesterol, lipid disorders, and breathing disorders. Right now, these drugs must get into the liver to work, but otherwise the future treatment possibilities are wide open, according to Dr. Zamore.
"It doesn't have to be a genetic disease," he says. "In theory, it doesn't have to be just one gene, although I don't think anyone knows how many you could target at once. There is no precedent for targeting two."

Dr. Phillip Zamore, chair of the RNA Therapeutics Institute at the University of Massachusetts Medical School.
(Courtesy of Dr. Zamore)
Alnylam, the leading company in RNAi therapeutics, plans to strategically design other new drugs based on what they have learned from this first trial – "so with each successive experience, with designing and testing, you get better at making more drugs. In a way, that's never happened before...This is a lot more efficient of a way to make drugs in the future."
And unlike gene therapy, in which a patient's own genetic code is permanently altered, this approach does not cause permanent genetic changes. Patients can stop taking it like any other drug, and its effects will vanish.
How Is Steve?
Last February, Steve started on the drug. He was granted early access since it is not yet FDA-approved and is still considered experimental. Every 21 days, he has received an IV infusion that causes some minor side effects, like headaches and facial flushing.
"The good news is, since I started on the drug, I don't see any more deterioration other than my speech."
So far, it seems to be effective. He's gained back 20 pounds, and though his enunciation is still a bit slurred, he says that his neuropathy has stopped. He plans to continue the treatment for the rest of his life.
"The good news is, since I started on the drug, I don't see any more deterioration other than my speech," he says. "I think the drug is working, but would I have continued to deteriorate without the drug? I'm not really sure."
Dr. Polydefkis jumps in with a more confident response: "If you ask me, I would say 100 percent he would have kept progressing at a fairly rapid pace without the drug. When Steve says the neuropathy has stopped, that's music to my ears."
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
“Synthetic Embryos”: The Wrong Term For Important New Research
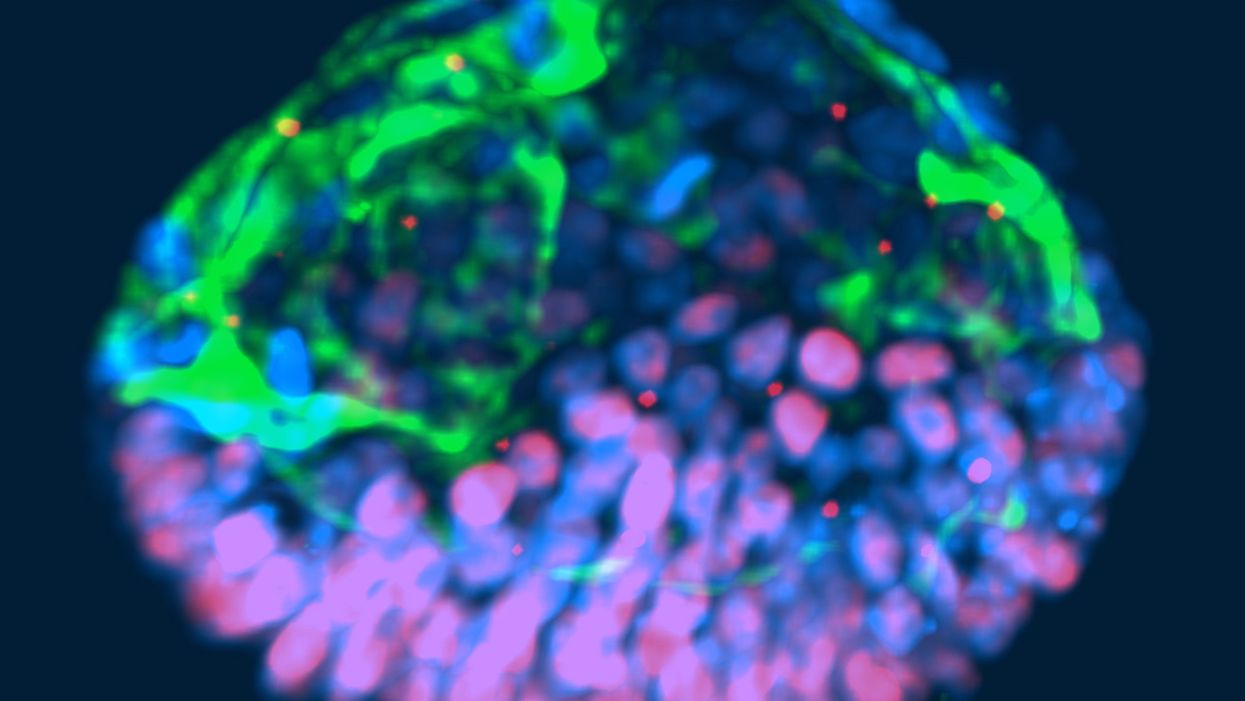
This fluorescent image shows a representative post-implantation amniotic sac embroid.
As a subject of research, an unusual degree of consensus appears to exist among scientists, politicians and the public about human embryos being deserving of special considerations. But what those special considerations should be is less clear. And this is where the subject becomes contentious and opinions diverge because, somewhat surprisingly, what really represents a human embryo has so far not been resolved.
"Prior to implantation, embryos must be given a different level of reverence than after implantation."
In 2002, Howard W. Jones Jr., widely considered the "father" of in vitro fertilization (IVF) in the U.S., argued in a widely acclaimed article titled "What is an embryo?" that a precondition for the definition of a human embryo was successful implantation. Only once implantation established a biological unit between embryo and mother, could a relatively small number of human cells be considered a human embryo.
Because he felt strongly that human embryos, indeed, deserve special considerations, and should receive those during IVF, he pointed out that, even inside a woman's body, most human embryos (in contrast to other species) never implant and, therefore, are never given a chance at human life. Consequently, he reasoned that prior to implantation, embryos must be given a different level of reverence than after implantation.
"One cannot help but wonder about the fog of misconceptions and misrepresentations that still surrounds what an embryo is."
This difference, he felt, should also be reflected in scientific language, proposing that embryos prior to implantation in daily IVF practice be called "pre-embryos," with the term "embryo" reserved for post-implantation-stage embryos. Then still unknown to Jones, recent research findings support this viewpoint, since genetic profiles of pre- and post-implantation stage embryos greatly differ.
In an analogy to nature, which in humans allows implantation of only a small minority of naturally generated pre-embryos, IVF centers around the world routinely discard large numbers of pre-embryos, judged inadequate for producing normal pregnancies. Jones' suggestion that only post-implantation embryos should be considered embryos deserving of special considerations, therefore, not only appears prescient and considerate of current IVF practices, but grounded in scientific reality. One, therefore, cannot help but wonder about the fog of misconceptions and misrepresentations that still surrounds what an embryo is.
"Much of the regulatory environment surrounding research on human embryos is guided by emotions rather than science and logical thinking."
In 1984, a British ethics committee issued the Warnock Report, which still today prohibits scientists worldwide from studying human embryos in a lab beyond 14 days from fertilization or past formation of the so-called primitive streak, whichever comes first. Well-meaning in its day, its intent was to apply special considerations to human pre-embryos by protecting them from the potential of "feeling pain," once the primitive streak arose on day-15 of development. Formation of the primitive streak signifies a process known as gastrulation, when a subset of cells from the inner cell mass of the pre-embryo are transformed into the three germ layers that comprise all tissues of the developing embryo: The ectoderm, which gives rise to the nervous system; the mesoderm, which gives rise to the circulatory system, muscle, and kidneys; and the endoderm which gives rise to the interior lining of the digestive and respiratory tracts, among other tissues.
That pre-embryos may feel pain at that stage of development was far-fetched in 1984; in view of what we have learned about early human embryology in the 33 years since, it remains untenable today. And, yet, scientists all over the world remain bound by the ethical constraints imposed by the Warnock Report.
A similar ethical paradox exists today for guidelines affecting huge numbers of so-called "abandoned" cryopreserved embryos, often stored ad infinitum in IVF centers all over the world. These are pre-embryos, whose "parents" are no longer responsive to queries from their IVF centers. Current U.S. guidelines allow the disposal of such pre-embryos but prohibit their use in research that may benefit mankind. One, however, wonders whether disposal of huge numbers of abandoned embryos is really more ethical than their use in potentially life-saving human research?
That much of the regulatory environment surrounding research on human embryos is, indeed, guided by emotions rather than science and logical thinking, is also demonstrated by recently expressed concern about so-called "artificial" or "synthetic" embryos. Though both of these terms suggest impending ability to create human embryos from synthetic building blocks, this is not what these terms are meant to describe (such abilities also are not on the horizon). They also do not describe abilities to create gametes (i.e., eggs and sperm) from somatic cells by reprogramming adult peripheral cells, which has already been successfully done in mice by Japanese investigators, leading to the creation of healthy embryos and births and three generations of healthy pubs. Such an approach is at least conceivable as an upcoming infertility treatment.
"A team of biologists and engineers at the University of Michigan recently received media attention after creating organoids from embryonic stem cells that resembled human embryos."
What all of this noise is really about is the discovery that, as several Rockefeller University investigators recently noted, "Cells have an intrinsic ability to self-assemble and self-organize into complex and functional tissues and organs." Investigators have taken advantage of this ability by creating in the lab so-called "organoids" from accumulations of individual embryonic stem cells. They are defined by three characteristics: (i) they contain a variety of cell types and tissue layers, all typical for a given organ; (ii) these cells are organized similarly to their organization in a specific organ; and (iii) the organoid mimics functions of the organ.
Several other biologists from the Cincinnati Children Hospital Medical Center recently noted that in the last five years, quite a variety of human stem cell-derived organoids, including all three germ layers, have been generated by different research groups around the world, thereby establishing new human model systems that can be used outside the body, in a dish, to investigate otherwise difficult-to-approach organs. Interestingly, they can also be used to investigate early stages of human embryological development.
A team of biologists and engineers at the University of Michigan recently received media attention after creating organoids from embryonic stem cells that resembled human embryos and, therefore, were given the name "embroids." Though clearly not embryos (the only thing they had in common with human embryos were cell types), they were nevertheless awarded in at least one article the identity of "artificial embryos," which "no one knows how to handle." As Howard Jones so correctly noted, with the word embryo often comes undeserved reverence.
"Any association with the term "embryo" should be avoided; it is not only misleading and irresponsible but scientifically incorrect."
Artificial embryos, therefore, do not exist. Organoids that resemble embryos (i.e., "embroids"), while potentially very useful research objects in studies of early human embryonic cell organization and lineage development, are not embryos--not even pre-embryos. Special considerations for "artificial" or "synthetic" embryos, as recently advocated by some scientists, therefore, appear ethically undeserved. How misdirected and forced some of these efforts are is probably best demonstrated by a recent publication in which a group of Harvard University investigators proposed the term "synthetic human entities with embryo-like features" or SHEEFS" in place of "organoids." Preferably, however, in describing these laboratory-created entities, any association with the term "embryo" should be avoided. It is not only misleading and irresponsible but scientifically incorrect.
Clinical reproductive medicine and reproductive biology, for valid ethical reasons, but also because of myths, misperceptions and, sometimes, outright misrepresentations of facts for political reasons, are under more public scrutiny than most other science areas. Yet, at least in the realm of biomedical research, nothing appears more important than better understanding the first few days of human embryo development. A recent study involving genetic editing of human embryos, reported by British investigators in Nature, once again confirmed what biologist have known for some time: No animal model faithfully recapitulates most of human developmental origins. The most important secrets nature still has to tell us, will not be revealed through mouse or other animal studies. We will discover them only through the study of early-stage human embryos – and we, therefore, should not limit the use of lab-grown organoids to help further that research.
Understanding early human development "will not only greatly enhance the biological understanding of our species; but also will open groundbreaking new therapeutic options in all areas of medicine."
As Howard Jones intuitively noticed, words matter. Appropriate and uniformly accepted definitions and terms are not only essential for scientific communications but, within the context of human reproduction, often elicit strong emotional reactions, and are easily misappropriated by those opposed to most interventions into human reproduction.
Who does not recall the early days of IVF in the late 1970s, when even reputable news outlets raised the specter of Frankenstein monsters created through the IVF process? Millions of IVF births later, a Nobel Prize in Medicine and Physiology was in 2010 finally awarded to the biologist Robert Edwards who, together with the gynecologist Patrick Steptoe, reported the first live birth through IVF on July 25, 1978. Many more awards are still waiting for recipients who through the study of early human embryo development will discover how cell fate is determined and cells acquire highly specific functions; how rapid cell proliferation takes place and, when required, stops; why chromosomal abnormalities are so common in early stage embryos and what their function may be.
Those who will discover these and many other important answers, will not only greatly enhance the biological understanding of our species; but also will open groundbreaking new therapeutic options in all areas of medicine. Learning how to control cell proliferation, for example, will likely revolutionize cancer therapy; I started my research career in biology with a study published in 1980 of "common denominators of pregnancy and malignancy." If regulatory prohibitions are not allowed to interfere in rapidly progressing research opportunities involving organoids and pre-embryos, we will, finally, see the circle closing, with the most rewarding benefits for mankind ever achieved through biological research.
Editor's Note: Read a different viewpoint here written by one of the world's top experts on the ethics of stem cell research.