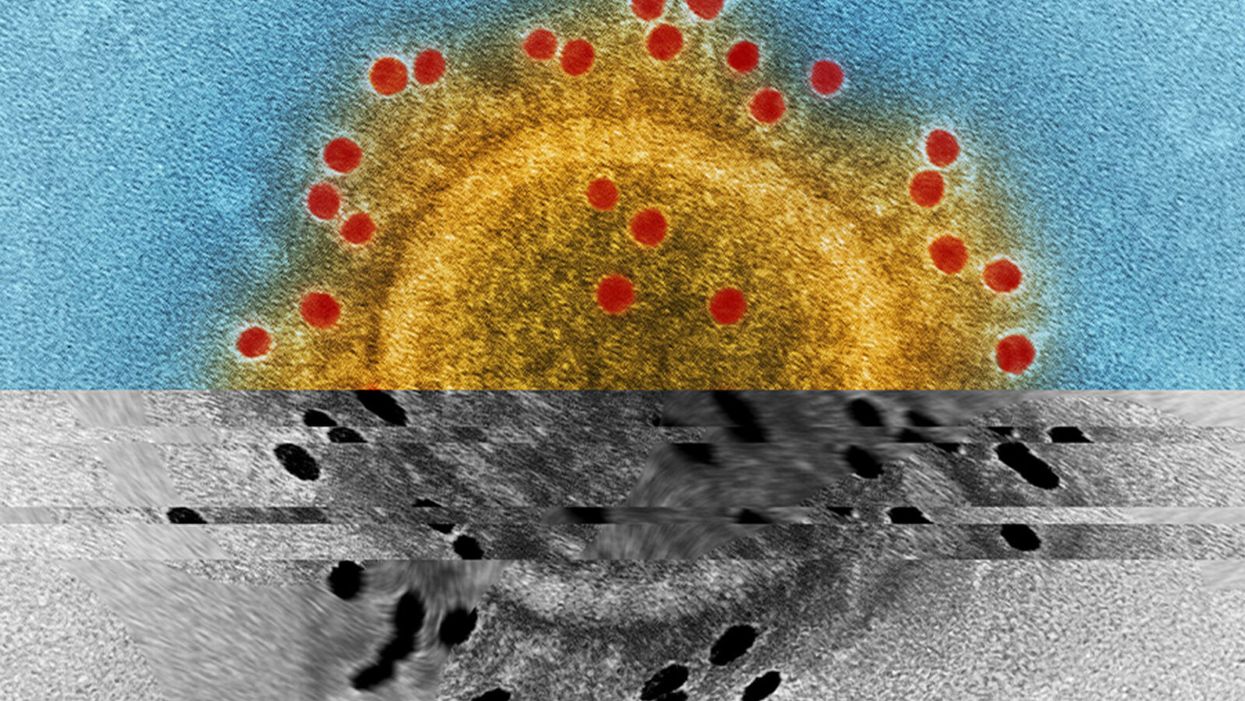
Why Haven’t Researchers Developed an HIV Vaccine or Cure Yet?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A blood test for analysis of HIV.
Last week, top experts on HIV/AIDS convened in Amsterdam for the 22nd International AIDS conference, and the mood was not great. Even though remarkable advances in treating HIV have led to effective management for many people living with the disease, and its overall incidence has declined, there are signs that the virus could make a troubling comeback.
"In a perfect world, we'd get a vaccine like the HPV vaccine that was 100% effective and I think that's ultimately what we're going to strive for."
Growing resistance to current HIV drugs, a population boom in Sub-Saharan Africa, and insufficient public health resources are all poised to contribute to a second AIDS pandemic, according to published reports.
Already, the virus is nowhere near under control. Though the infection rate has declined 47 percent since its peak in 1996, last year 1.8 million people became newly infected with HIV around the world, and 37 million people are currently living with it. About 1 million people die of AIDS every year, making it the fourth biggest killer in low-income countries.
Leapsmag Editor-in-Chief Kira Peikoff reached out to Dr. Carl Dieffenbach, Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, to find out what the U.S. government is doing to develop an HIV vaccine and cure. This interview has been edited and condensed for clarity.
What is the general trajectory of research in HIV/AIDS today?
We can break it down to two specific domains: focus on treatment and cure, and prevention.
Let's start with people living with HIV. This is the area where we've had the most success over the past 30 plus years, because we've taken a disease that was essentially a death sentence and converted it through the development of medications to a treatable chronic disease.
The second half of this equation is, can we cure or create a functional cure for people living with HIV? And the definition of functional cure would be the absence of circulating virus in the body in the absence of therapy. Essentially the human body would control the HIV infection within the individual. That is a much more, very early research stage of discovery. There are some interesting signals but it's still in need of innovation.
I'd like to make a contrast between what we are able to do with a virus called Hepatitis C and what we can do with the virus HIV. Hep C, with 12 weeks of highly active antiviral therapy, we can cure 95 to 100% of infections. With HIV, we cannot do that. The difference is the behavior of the virus. HIV integrates into the host's genome. Hep C is an RNA virus that stays in the cytoplasm of the cell and never gets into the DNA.
On the prevention side, we have two strategies: The first is pre-exposure prophylaxis. Then of course, we have the need for a safe, effective and durable HIV vaccine, which is a very active area of discovery. We've had some spectacular success with RV144, and we're following up on that success, and other vaccines are in the pipeline. Whether they are sufficient to provide the level of durability and activity is not yet clear, but progress has been made and there's still the need for innovation.
The most important breakthrough in the past 5 to 10 years has been the discovery of broad neutralizing monoclonal antibodies. They are proteins that the body makes, and not everybody who's HIV infected makes these antibodies, but we've been able to clone out these antibodies from certain individuals that are highly potent, and when used either singly or in combination, can truly neutralize the vast majority of HIV strains. Can those be used by themselves as treatment or as prevention? That is the question.
Can you explain more about RV144 and why you consider it a success?
Prior to RV144, we had run a number of vaccine studies and nothing had ever statistically shown to be protective. RV144 showed a level of efficacy of about 31 percent, which was statistically significant. Not enough to take forward into other studies, but it allowed us to generate some ideas about why this worked, go back to the drawing board, and redesign the immunogens to optimize and test the next generation for this vaccine. We just recently opened that new study, the follow-up to RV144, called HVTN702. That's up and enrolling and moving along quite nicely.

Carl Dieffenbach, Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases
(Courtesy)
Where is that enrolling?
Primarily in Sub-Saharan Africa and South Africa.
When will you expect to see signals from that?
Between 2020 and 2021. It's complicated because the signal also takes into account the durability. After a certain time of vaccination, we're going to count up endpoints.
How would you explain the main scientific obstacle in the way of creating a very efficacious HIV vaccine?
Simply put, it's the black box of the human immune system. HIV employs a shield technology, and the virus is constantly changing its shield to protect itself, but there are some key parts of the virus that it cannot shield, so that's the trick – to be able to target that.
So, you're trying to find the Achilles' Heel of the virus?
Exactly. To make a flu vaccine or a Zika vaccine or even an Ebola vaccine, the virus is a little bit more forthcoming with the target. In HIV, the virus does everything in its power to hide the target, so we're dealing with a well-adapted [adversary] that actively avoids neutralization. That's the scientific challenge we face.
What's next?
On the vaccine side, we are currently performing, in collaboration with partners, two vaccine trials – HVTN702, which we talked about, and another one called 705. If either of those are highly successful, they would both require an additional phase 3 clinical trial before they could be licensed. This is an important but not final step. Then we would move into scale up to global vaccination. Those conversations have begun but they are not very far along and need additional attention.
What percent of people in the current trials would need to be protected to move on to phase 3?
Between 50 and 60 percent. That comes with this question of durability: how long does the vaccine last?
It also includes, can we simplify the vaccine regimen? The vaccines we're testing right now are multiple shots over a period of time. Can we get more like the polio or smallpox vaccine, a shot with a booster down the road?
We're dealing with sovereign nations. We're doing this in partnership, not as helicopter-type researchers.
If these current trials pan out, do you think kids in the developed world will end up getting an HIV vaccine one day? Or just people in-at risk areas?
That's a good question. I don't have an answer to that. In a perfect world, we'd get a vaccine like the HPV vaccine that was 100% effective and I think that's ultimately what we're going to strive for. That's where that second or third generation of vaccines that trigger broad neutralizing antibodies come in.
With any luck at all, globally, the combination of antiretroviral treatment, pre-exposure prophylaxis and other prevention and treatment strategies will lower the incidence rate where the HIV pandemic continues to wane, and we will then be able to either target the vaccine or roll it out in a way that is both cost effective and destigmatizing.
And also, what does the country want? We're dealing with sovereign nations. We're doing this in partnership, not as helicopter-type researchers.
How close do you think we are globally to eradicating HIV infections?
Eradication's a big word. It means no new infections. We are nowhere close to eradicating HIV. Whether or not we can continue to bend the curve on the epidemic and have less infections so that the total number of people continues to decline over time, I think we can achieve that if we had the political will. And that's not just the U.S. political will. That's the will of the world. We have the tools, albeit they're not perfect. But that's where a vaccine that is efficacious and simple to deliver could be the gamechanger.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Pseudoscience Is Rampant: How Not to Fall for It
A metaphorical rendering of scientific truth gone awry.
Whom to believe?
The relentless and often unpredictable coronavirus (SARS-CoV-2) has, among its many quirky terrors, dredged up once again the issue that will not die: science versus pseudoscience.
How does one learn to spot the con without getting a Ph.D. and spending years in a laboratory?
The scientists, experts who would be the first to admit they are not infallible, are now in danger of being drowned out by the growing chorus of pseudoscientists, conspiracy theorists, and just plain troublemakers that seem to be as symptomatic of the virus as fever and weakness.
How is the average citizen to filter this cacophony of information and misinformation posing as science alongside real science? While all that noise makes it difficult to separate the real stuff from the fakes, there is at least one positive aspect to it all.
A famous aphorism by one Charles Caleb Colton, a popular 19th-century English cleric and writer, says that "imitation is the sincerest form of flattery."
The frauds and the paranoid conspiracy mongers who would perpetrate false science on a susceptible public are at least recognizing the value of science—they imitate it. They imitate the ways in which science works and make claims as if they were scientists, because even they recognize the power of a scientific approach. They are inadvertently showing us how much we value science. Unfortunately they are just shabby counterfeits.
Separating real science from pseudoscience is not a new problem. Philosophers, politicians, scientists, and others have been worrying about this perhaps since science as we know it, a science based entirely on experiment and not opinion, arrived in the 1600s. The original charter of the British Royal Society, the first organized scientific society, stated that at their formal meetings there would be no discussion of politics, religion, or perpetual motion machines.
The first two of those for the obvious purpose of keeping the peace. But the third is interesting because at that time perpetual motion machines were one of the main offerings of the imitators, the bogus scientists who were sure that you could find ways around the universal laws of energy and make a buck on it. The motto adopted by the society was, and remains, Nullius in verba, Latin for "take nobody's word for it." Kind of an early version of Missouri's venerable state motto: "Show me."
You might think that telling phony science from the real thing wouldn't be so difficult, but events, historical and current, tell a very different story—often with tragic outcomes. Just one terrible example is the estimated 350,000 additional HIV deaths in South Africa directly caused by the now-infamous conspiracy theories of their own elected President no less (sound familiar?). It's surprisingly easy to dress up phony science as the real thing by simply adopting, or appearing to adopt, the trappings of science.
Thus, the anti-vaccine movement claims to be based on suspicion of authority, beginning with medical authority in this case, stemming from the fraudulent data published by the now-disgraced Andrew Wakefield, an English gastroenterologist. And it's true that much of science is based on suspicion of authority. Science got its start when the likes of Galileo and Copernicus claimed that the Church, the State, even Aristotle, could no longer be trusted as authoritative sources of knowledge.
But Galileo and those who followed him produced alternative explanations, and those alternatives were based on data that arose independently from many sources and generated a great deal of debate and, most importantly, could be tested by experiments that could prove them wrong. The anti-vaccine movement imitates science, still citing the discredited Wakefield report, but really offers nothing but suspicion—and that is paranoia, not science.
Similarly, there are those who try to cloak their nefarious motives in the trappings of science by claiming that they are taking the scientific posture of doubt. Science after all depends on doubt—every scientist doubts every finding they make. Every scientist knows that they can't possibly foresee all possible instances or situations in which they could be proven wrong, no matter how strong their data. Einstein was doubted for two decades, and cosmologists are still searching for experimental proofs of relativity. Science indeed progresses by doubt. In science revision is a victory.
But the imitators merely use doubt to suggest that science is not dependable and should not be used for informing policy or altering our behavior. They claim to be taking the legitimate scientific stance of doubt. Of course, they don't doubt everything, only what is problematic for their individual enterprises. They don't doubt the value of blood pressure medicine to control their hypertension. But they should, because every medicine has side effects and we don't completely understand how blood pressure is regulated and whether there may not be still better ways of controlling it.
But we use the pills we have because the science is sound even when it is not completely settled. Ask a hypertensive oil executive who would like you to believe that climate science should be ignored because there are too many uncertainties in the data, if he is willing to forgo his blood pressure medicine—because it, too, has its share of uncertainties and unwanted side effects.
The apparent success of pseudoscience is not due to gullibility on the part of the public. The problem is that science is recognized as valuable and that the imitators are unfortunately good at what they do. They take a scientific pose to gain your confidence and then distort the facts to their own purposes. How does one learn to spot the con without getting a Ph.D. and spending years in a laboratory?
"If someone claims to have the ultimate answer or that they know something for certain, the only thing for sure is that they are trying to fool you."
What can be done to make the distinction clearer? Several solutions have been tried—and seem to have failed. Radio and television shows about the latest scientific breakthroughs are a noble attempt to give the public a taste of good science, but they do nothing to help you distinguish between them and the pseudoscience being purveyed on the neighboring channel and its "scientific investigations" of haunted houses.
Similarly, attempts to inculcate what are called "scientific habits of mind" are of little practical help. These habits of mind are not so easy to adopt. They invariably require some amount of statistics and probability and much of that is counterintuitive—one of the great values of science is to help us to counter our normal biases and expectations by showing that the actual measurements may not bear them out.
Additionally, there is math—no matter how much you try to hide it, much of the language of science is math (Galileo said that). And half the audience is gone with each equation (Stephen Hawking said that). It's hard to imagine a successful program of making a non-scientifically trained public interested in adopting the rigors of scientific habits of mind. Indeed, I suspect there are some people, artists for example, who would be rightfully suspicious of changing their thinking to being habitually scientific. Many scientists are frustrated by the public's inability to think like a scientist, but in fact it is neither easy nor always desirable to do so. And it is certainly not practical.
There is a more intuitive and simpler way to tell the difference between the real thing and the cheap knock-off. In fact, it is not so much intuitive as it is counterintuitive, so it takes a little bit of mental work. But the good thing is it works almost all the time by following a simple, if as I say, counterintuitive, rule.
True science, you see, is mostly concerned with the unknown and the uncertain. If someone claims to have the ultimate answer or that they know something for certain, the only thing for sure is that they are trying to fool you. Mystery and uncertainty may not strike you right off as desirable or strong traits, but that is precisely where one finds the creative solutions that science has historically arrived at. Yes, science accumulates factual knowledge, but it is at its best when it generates new and better questions. Uncertainty is not a place of worry, but of opportunity. Progress lives at the border of the unknown.
How much would it take to alter the public perception of science to appreciate unknowns and uncertainties along with facts and conclusions? Less than you might think. In fact, we make decisions based on uncertainty every day—what to wear in case of 60 percent chance of rain—so it should not be so difficult to extend that to science, in spite of what you were taught in school about all the hard facts in those giant textbooks.
You can believe science that says there is clear evidence that takes us this far… and then we have to speculate a bit and it could go one of two or three ways—or maybe even some way we don't see yet. But like your blood pressure medicine, the stuff we know is reliable even if incomplete. It will lower your blood pressure, no matter that better treatments with fewer side effects may await us in the future.
Unsettled science is not unsound science. The honesty and humility of someone who is willing to tell you that they don't have all the answers, but they do have some thoughtful questions to pursue, are easy to distinguish from the charlatans who have ready answers or claim that nothing should be done until we are an impossible 100-percent sure.
Imitation may be the sincerest form of flattery.
The problem, as we all know, is that flattery will get you nowhere.
[Editor's Note: This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
Henrietta Lacks' Cells Enabled Medical Breakthroughs. Is It Time to Finally Retire Them?
Henrietta Lacks, (1920-1951) unknowingly had her cells cultured and used in medical research.
For Victoria Tokarz, a third-year PhD student at the University of Toronto, experimenting with cells is just part of a day's work. Tokarz, 26, is studying to be a cell biologist and spends her time inside the lab manipulating muscle cells sourced from rodents to try to figure out how they respond to insulin. She hopes this research could someday lead to a breakthrough in our understanding of diabetes.
"People like to use HeLa cells because they're easy to use."
But in all her research, there is one cell culture that Tokarz refuses to touch. The culture is called HeLa, short for Henrietta Lacks, named after the 31-year-old tobacco farmer the cells were stolen from during a tumor biopsy she underwent in 1951.
"In my opinion, there's no question or experiment I can think of that validates stealing from and profiting off of a black woman's body," Tokarz says. "We're not talking about a reagent we created in a lab, a mixture of some chemicals. We're talking about a human being who suffered indescribably so we could profit off of her misfortune."
Lacks' suffering is something that, until recently, was not widely known. Born to a poor family in Roanoke, VA, Lacks was sent to live with her grandfather on the family tobacco farm at age four, shortly after the death of her mother. She gave birth to her first child at just fourteen, and two years later had another child with profound developmental disabilities. Lacks married her first cousin, David, in 1941 and the family moved to Maryland where they had three additional children.
But the real misfortune came in 1951, when Lacks told her cousins that she felt a hard "knot" in her womb. When Lacks went to Johns Hopkins hospital to have the knot examined, doctors discovered that the hard lump Henrietta felt was a rapidly-growing cervical tumor.
Before the doctors treated the tumor – inserting radium tubes into her vagina, in the hopes they could kill the cancer, Lacks' doctors clipped two tissue samples from her cervix, without Lacks' knowledge or consent. While it's considered widely unethical today, taking tissue samples from patients was commonplace at the time. The samples were sent to a cancer researcher at Johns Hopkins and Lacks continued treatment unsuccessfully until she died a few months later of metastatic cancer.
Lacks' story was not over, however: When her tissue sample arrived at the lab of George Otto Gey, the Johns Hopkins cancer researcher, he noticed that the cancerous cells grew at a shocking pace. Unlike other cell cultures that would die within a day or two of arriving at the lab, Lacks' cells kept multiplying. They doubled every 24 hours, and to this day, have never stopped.
Scientists would later find out that this growth was due to an infection of Human Papilloma Virus, or HPV, which is known for causing aggressive cancers. Lacks' cells became the world's first-ever "immortalized" human cell line, meaning that as long as certain environmental conditions are met, the cells can replicate indefinitely. Although scientists have cultivated other immortalized cell lines since then, HeLa cells remain a favorite among scientists due to their resilience, Tokarz says.
"People like to use HeLa cells because they're easy to use," Tokarz says. "They're easy to manipulate, because they're very hardy, and they allow for transection, which means expressing a protein in a cell that's not normally there. Other cells, like endothelial cells, don't handle those manipulations well."
Once the doctors at Johns Hopkins discovered that Lacks' cells could replicate indefinitely, they started shipping them to labs around the world to promote medical research. As they were the only immortalized cell line available at the time, researchers used them for thousands of experiments — some of which resulted in life-saving treatments. Jonas Salk's polio vaccine, for example, was manufactured using HeLa cells. HeLa cell research was also used to develop a vaccine for HPV, and for the development of in vitro fertilization and gene mapping. Between 1951 and 2018, HeLa cells have been cited in over 110,000 publications, according to a review from the National Institutes of Health.
But while some scientists like Tokarz are thankful for the advances brought about by HeLa cells, they still believe it's well past time to stop using them in research.
"Am I thankful we have a polio vaccine? Absolutely. Do I resent the way we came to have that vaccine? Absolutely," Tokarz says. "We could have still arrived at those same advances by treating her as the human being she is, not just a specimen."
Ethical considerations aside, HeLa is no longer the world's only available cell line – nor, Tokarz argues, are her cells the most suitable for every type of research. "The closer you can get to the physiology of the thing you're studying, the better," she says. "Now we have the ability to use primary cells, which are isolated from a person and put right into the culture dish, and those don't have the same mutations as cells that have been growing for 20 years. We didn't have the expertise to do that initially, but now we do."
Raphael Valdivia, a professor of molecular genetics and microbiology at Duke University School of Medicine, agrees that HeLa cells are no longer optimal for most research. "A lot of scientists are moving away from HeLa cells because they're so unstable," he says. "They mutate, they rearrange chromosomes to become adaptive, and different batches of cells evolve separately from each other. The HeLa cells in my lab are very different than the ones down the hall, and that means sometimes we can't replicate our results. We have to go back to an earlier batch of cells in the freezer and re-test."
Still, the idea of retiring the cells completely doesn't make sense, Valdivia says: "To some extent, you're beholden to previous research. You need to be able to confirm findings that happen in earlier studies, and to do that you need to use the same cell line that other researchers have used."
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice."
"The way in which the cells were taken – without patient consent – is completely inappropriate," says Yann Joly, associate professor at the Faculty of Medicine in Toronto and Research Director at the Centre of Genomics and Policy. "The question now becomes, what can we do about it now? What are our options?"
While scientists are not able to erase what was done to Henrietta Lacks, Joly argues that retiring her cells is also non-consensual, assuming – maybe incorrectly – what Henrietta would have wanted, without her input. Additionally, Joly points out that other immortalized human cell lines are fraught with what some people consider to be ethical concerns as well, such as the human embryonic kidney cell line, commonly referred to as HEK-293, that was derived from an aborted female fetus. "Just because you're using another kind of cell doesn't mean it's devoid of ethical issue," he says.
Seemingly, the one thing scientists can agree on is that Henrietta Lacks was mistreated by the medical community. But even so, retiring her cells from medical research is not an obvious solution. Scientists are now using HeLa cells to better understand how the novel coronavirus affects humans, and this knowledge will inform how researchers develop a COVID-19 vaccine.
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice," Joly says. "If [ethics] were that easy, nobody would need to teach it."