Why Haven’t Researchers Developed an HIV Vaccine or Cure Yet?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A blood test for analysis of HIV.
Last week, top experts on HIV/AIDS convened in Amsterdam for the 22nd International AIDS conference, and the mood was not great. Even though remarkable advances in treating HIV have led to effective management for many people living with the disease, and its overall incidence has declined, there are signs that the virus could make a troubling comeback.
"In a perfect world, we'd get a vaccine like the HPV vaccine that was 100% effective and I think that's ultimately what we're going to strive for."
Growing resistance to current HIV drugs, a population boom in Sub-Saharan Africa, and insufficient public health resources are all poised to contribute to a second AIDS pandemic, according to published reports.
Already, the virus is nowhere near under control. Though the infection rate has declined 47 percent since its peak in 1996, last year 1.8 million people became newly infected with HIV around the world, and 37 million people are currently living with it. About 1 million people die of AIDS every year, making it the fourth biggest killer in low-income countries.
Leapsmag Editor-in-Chief Kira Peikoff reached out to Dr. Carl Dieffenbach, Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, to find out what the U.S. government is doing to develop an HIV vaccine and cure. This interview has been edited and condensed for clarity.
What is the general trajectory of research in HIV/AIDS today?
We can break it down to two specific domains: focus on treatment and cure, and prevention.
Let's start with people living with HIV. This is the area where we've had the most success over the past 30 plus years, because we've taken a disease that was essentially a death sentence and converted it through the development of medications to a treatable chronic disease.
The second half of this equation is, can we cure or create a functional cure for people living with HIV? And the definition of functional cure would be the absence of circulating virus in the body in the absence of therapy. Essentially the human body would control the HIV infection within the individual. That is a much more, very early research stage of discovery. There are some interesting signals but it's still in need of innovation.
I'd like to make a contrast between what we are able to do with a virus called Hepatitis C and what we can do with the virus HIV. Hep C, with 12 weeks of highly active antiviral therapy, we can cure 95 to 100% of infections. With HIV, we cannot do that. The difference is the behavior of the virus. HIV integrates into the host's genome. Hep C is an RNA virus that stays in the cytoplasm of the cell and never gets into the DNA.
On the prevention side, we have two strategies: The first is pre-exposure prophylaxis. Then of course, we have the need for a safe, effective and durable HIV vaccine, which is a very active area of discovery. We've had some spectacular success with RV144, and we're following up on that success, and other vaccines are in the pipeline. Whether they are sufficient to provide the level of durability and activity is not yet clear, but progress has been made and there's still the need for innovation.
The most important breakthrough in the past 5 to 10 years has been the discovery of broad neutralizing monoclonal antibodies. They are proteins that the body makes, and not everybody who's HIV infected makes these antibodies, but we've been able to clone out these antibodies from certain individuals that are highly potent, and when used either singly or in combination, can truly neutralize the vast majority of HIV strains. Can those be used by themselves as treatment or as prevention? That is the question.
Can you explain more about RV144 and why you consider it a success?
Prior to RV144, we had run a number of vaccine studies and nothing had ever statistically shown to be protective. RV144 showed a level of efficacy of about 31 percent, which was statistically significant. Not enough to take forward into other studies, but it allowed us to generate some ideas about why this worked, go back to the drawing board, and redesign the immunogens to optimize and test the next generation for this vaccine. We just recently opened that new study, the follow-up to RV144, called HVTN702. That's up and enrolling and moving along quite nicely.

Carl Dieffenbach, Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases
(Courtesy)
Where is that enrolling?
Primarily in Sub-Saharan Africa and South Africa.
When will you expect to see signals from that?
Between 2020 and 2021. It's complicated because the signal also takes into account the durability. After a certain time of vaccination, we're going to count up endpoints.
How would you explain the main scientific obstacle in the way of creating a very efficacious HIV vaccine?
Simply put, it's the black box of the human immune system. HIV employs a shield technology, and the virus is constantly changing its shield to protect itself, but there are some key parts of the virus that it cannot shield, so that's the trick – to be able to target that.
So, you're trying to find the Achilles' Heel of the virus?
Exactly. To make a flu vaccine or a Zika vaccine or even an Ebola vaccine, the virus is a little bit more forthcoming with the target. In HIV, the virus does everything in its power to hide the target, so we're dealing with a well-adapted [adversary] that actively avoids neutralization. That's the scientific challenge we face.
What's next?
On the vaccine side, we are currently performing, in collaboration with partners, two vaccine trials – HVTN702, which we talked about, and another one called 705. If either of those are highly successful, they would both require an additional phase 3 clinical trial before they could be licensed. This is an important but not final step. Then we would move into scale up to global vaccination. Those conversations have begun but they are not very far along and need additional attention.
What percent of people in the current trials would need to be protected to move on to phase 3?
Between 50 and 60 percent. That comes with this question of durability: how long does the vaccine last?
It also includes, can we simplify the vaccine regimen? The vaccines we're testing right now are multiple shots over a period of time. Can we get more like the polio or smallpox vaccine, a shot with a booster down the road?
We're dealing with sovereign nations. We're doing this in partnership, not as helicopter-type researchers.
If these current trials pan out, do you think kids in the developed world will end up getting an HIV vaccine one day? Or just people in-at risk areas?
That's a good question. I don't have an answer to that. In a perfect world, we'd get a vaccine like the HPV vaccine that was 100% effective and I think that's ultimately what we're going to strive for. That's where that second or third generation of vaccines that trigger broad neutralizing antibodies come in.
With any luck at all, globally, the combination of antiretroviral treatment, pre-exposure prophylaxis and other prevention and treatment strategies will lower the incidence rate where the HIV pandemic continues to wane, and we will then be able to either target the vaccine or roll it out in a way that is both cost effective and destigmatizing.
And also, what does the country want? We're dealing with sovereign nations. We're doing this in partnership, not as helicopter-type researchers.
How close do you think we are globally to eradicating HIV infections?
Eradication's a big word. It means no new infections. We are nowhere close to eradicating HIV. Whether or not we can continue to bend the curve on the epidemic and have less infections so that the total number of people continues to decline over time, I think we can achieve that if we had the political will. And that's not just the U.S. political will. That's the will of the world. We have the tools, albeit they're not perfect. But that's where a vaccine that is efficacious and simple to deliver could be the gamechanger.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Why you should (virtually) care
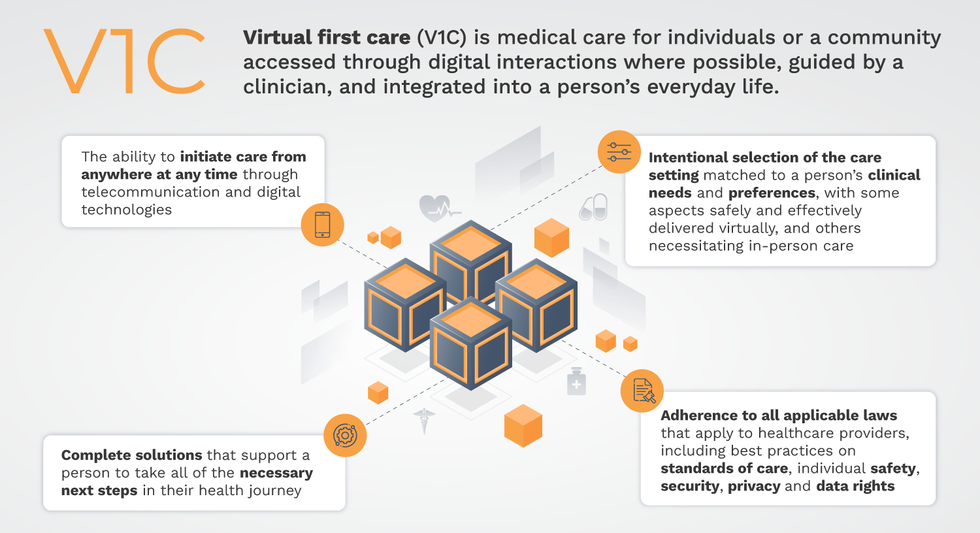
Virtual-first care, or V1C, could increase the quality of healthcare and make it more patient-centric by letting patients combine in-person visits with virtual options such as video for seeing their care providers.
As the pandemic turns endemic, healthcare providers have been eagerly urging patients to return to their offices to enjoy the benefits of in-person care.
But wait.
The last two years have forced all sorts of organizations to be nimble, adaptable and creative in how they work, and this includes healthcare providers’ efforts to maintain continuity of care under the most challenging of conditions. So before we go back to “business as usual,” don’t we owe it to those providers and ourselves to admit that business as usual did not work for most of the people the industry exists to help? If we’re going to embrace yet another period of change – periods that don’t happen often in our complex industry – shouldn’t we first stop and ask ourselves what we’re trying to achieve?
Certainly, COVID has shown that telehealth can be an invaluable tool, particularly for patients in rural and underserved communities that lack access to specialty care. It’s also become clear that many – though not all – healthcare encounters can be effectively conducted from afar. That said, the telehealth tactics that filled the gap during the pandemic were largely stitched together substitutes for existing visit-based workflows: with offices closed, patients scheduled video visits for help managing the side effects of their blood pressure medications or to see their endocrinologist for a quarterly check-in. Anyone whose children slogged through the last year or two of remote learning can tell you that simply virtualizing existing processes doesn’t necessarily improve the experience or the outcomes!
But what if our approach to post-pandemic healthcare came from a patient-driven perspective? We have a fleeting opportunity to advance a care model centered on convenient and equitable access that first prioritizes good outcomes, then selects approaches to care – and locations – tailored to each patient. Using the example of education, imagine how effective it would be if each student, regardless of their school district and aptitude, received such individualized attention.
That’s the idea behind virtual-first care (V1C), a new care model centered on convenient, customized, high-quality care that integrates a full suite of services tailored directly to patients’ clinical needs and preferences. This package includes asynchronous communication such as texting; video and other live virtual modes; and in-person options.
V1C goes beyond what you might think of as standard “telehealth” by using evidence-based protocols and tools that include traditional and digital therapeutics and testing, personalized care plans, dynamic patient monitoring, and team-based approaches to care. This could include spit kits mailed for laboratory tests and complementing clinical care with health coaching. V1C also replaces some in-person exams with ongoing monitoring, using sensors for more ‘whole person’ care.
Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
Established V1C healthcare providers such as Omada, Headspace, and Heartbeat Health, as well as emerging market entrants like Oshi, Visana, and Wellinks, work with a variety of patients who have complicated long-term conditions such as diabetes, heart failure, gastrointestinal illness, endometriosis, and COPD. V1C is comprehensive in ways that are lacking in digital health and its other predecessors: it has the potential to integrate multiple data streams, incorporate more frequent touches and check-ins over time, and manage a much wider range of chronic health conditions, improving lives and reducing disease burden now and in the future.
Recognizing the pandemic-driven interest in virtual care, significant energy and resources are already flowing fast toward V1C. Some of the world’s largest innovators jumped into V1C early on: Verily, Alphabet’s Life Sciences Company, launched Onduo in 2016 to disrupt the diabetes healthcare market, and is now well positioned to scale its solutions. Major insurers like Aetna and United now offer virtual-first plans to members, responding as organizations expand virtual options for employees. Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
That was the immediate impetus for IMPACT, a consortium of V1C companies, investors, payers and patients founded last year to ensure access to high-quality, evidence-based V1C. Developed by our team at the Digital Medicine Society (DiMe) in collaboration with the American Telemedicine Association (ATA), IMPACT has begun to explore key issues that include giving patients more integrated experiences when accessing both virtual and brick-and-mortar care.
Digital Medicine Society
V1C is not, nor should it be, virtual-only care. In this new era of hybrid healthcare, success will be defined by how well providers help patients navigate the transitions. How do we smoothly hand a patient off from an onsite primary care physician to, say, a virtual cardiologist? How do we get information from a brick-and-mortar to a digital portal? How do you manage dataflow while still staying HIPAA compliant? There are many complex regulatory implications for these new models, as well as an evolving landscape in terms of privacy, security and interoperability. It will be no small task for groups like IMPACT to determine the best path forward.
None of these factors matter unless the industry can recruit and retain clinicians. Our field is facing an unprecedented workforce crisis. Traditional healthcare is making clinicians miserable, and COVID has only accelerated the trend of overworked, disenchanted healthcare workers leaving in droves. Clinicians want more interactions with patients, and fewer with computer screens – call it “More face time, less FaceTime.” No new model will succeed unless the industry can more efficiently deploy its talent – arguably its most scarce and precious resource. V1C can help with alleviating the increasing burden and frustration borne by individual physicians in today’s status quo.
In healthcare, new technological approaches inevitably provoke no shortage of skepticism. Past lessons from Silicon Valley-driven fixes have led to understandable cynicism. But V1C is a different breed of animal. By building healthcare around the patient, not the clinic, V1C can make healthcare work better for patients, payers and providers. We’re at a fork in the road: we can revert back to a broken sick-care system, or dig in and do the hard work of figuring out how this future-forward healthcare system gets financed, organized and executed. As a field, we must find the courage and summon the energy to embrace this moment, and make it a moment of change.
Podcast: The future of brain health with Percy Griffin
Percy Griffin, director of scientific engagement for the Alzheimer’s Association, joins Leaps.org to discuss the present and future of the fight against dementia.
Today's guest is Percy Griffin, director of scientific engagement for the Alzheimer’s Association, a nonprofit that’s focused on speeding up research, finding better ways to detect Alzheimer’s earlier and other approaches for reducing risk. Percy has a doctorate in molecular cell biology from Washington University, he’s led important research on Alzheimer’s, and you can find the link to his full bio in the show notes, below.
Our topic for this conversation is the present and future of the fight against dementia. Billions of dollars have been spent by the National Institutes of Health and biotechs to research new treatments for Alzheimer's and other forms of dementia, but so far there's been little to show for it. Last year, Aduhelm became the first drug to be approved by the FDA for Alzheimer’s in 20 years, but it's received a raft of bad publicity, with red flags about its effectiveness, side effects and cost.
Meanwhile, 6.5 million Americans have Alzheimer's, and this number could increase to 13 million in 2050. Listen to this conversation if you’re concerned about your own brain health, that of family members getting older, or if you’re just concerned about the future of this country with experts predicting the number people over 65 will increase dramatically in the very near future.
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
4:40 - We talk about the parts of Percy’s life that led to him to concentrate on working in this important area.
6:20 - He defines Alzheimer's and dementia, and discusses the key elements of communicating science.
10:20 - Percy explains why the Alzheimer’s Association has been supportive of Aduhelm, even as others have been critical.
17:58 - We talk about therapeutics under development, which ones to be excited about, and how they could be tailored to a person's own biology.
24:25 - Percy discusses funding and tradeoffs between investing more money into Alzheimer’s research compared to other intractable diseases like cancer, and new opportunities to accelerate progress, such as ARPA-H, President Biden’s proposed agency to speed up health breakthroughs.
27:24 - We talk about the social determinants of brain health. What are the pros/cons of continuing to spend massive sums of money to develop new drugs like Aduhelm versus refocusing on expanding policies to address social determinants - like better education, nutritious food and safe drinking water - that have enabled some groups more than others to enjoy improved cognition late in life.
34:18 - Percy describes his top lifestyle recommendations for protecting your mind.
37:33 - Is napping bad for the brain?
39:39 - Circadian rhythm and Alzheimer's.
42:34 - What tests can people take to check their brain health today, and which biomarkers are we making progress on?
47:25 - Percy highlights important programs run by the Alzheimer’s Association to support advances.
Show links:
** After this episode was recorded, the Centers for Medicare and Medicaid Services affirmed its decision from last June to limit coverage of Aduhelm. More here.
- Percy Griffin's bio: https://www.alz.org/manh/events/alztalks/upcoming-...
- The Alzheimer's Association's Part the Cloud program: https://alz.org/partthecloud/about-us.asp
- The paradox of dementia rates decreasing: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7455342/
- The argument for focusing more resources on improving institutions and social processes for brain health: https://www.statnews.com/2021/09/23/the-brain-heal...
- Recent research on napping: https://www.ocregister.com/2022/03/25/alzheimers-s...
- The Alzheimer's Association helpline: https://www.alz.org/help-support/resources/helpline
- ALZConnected, a free online community for people affected by dementia https://www.alzconnected.org/
- TrialMatch for people with dementia and healthy volunteers to find clinical trials for Alzheimer's and other dementia: https://www.alz.org/alzheimers-dementia/research_p...

