Will Blockchain Technology Usher in a Healthcare Data Revolution?

Digital blockchain concept.
The hacker collective known as the Dark Overlord first surfaced in June 2016, when it advertised more than 600,000 patient files from three U.S. healthcare organizations for sale on the dark web. The group, which also attempted to extort ransom from its victims, soon offered another 9 million records pilfered from health insurance companies and provider networks across the country.
Since 2009, federal regulators have counted nearly 5,000 major data breaches in the United States alone, affecting some 260 million individuals.
Last October, apparently seeking publicity as well as cash, the hackers stole a trove of potentially scandalous data from a celebrity plastic surgery clinic in London—including photos of in-progress genitalia- and breast-enhancement surgeries. "We have TBs [terabytes] of this shit. Databases, names, everything," a gang representative told a reporter. "There are some royal families in here."
Bandits like these are prowling healthcare's digital highways in growing numbers. Since 2009, federal regulators have counted nearly 5,000 major data breaches in the United States alone, affecting some 260 million individuals. Although hacker incidents represent less than 20 percent of the total breaches, they account for almost 80 percent of the affected patients. Such attacks expose patients to potential blackmail or identity theft, enable criminals to commit medical fraud or file false tax returns, and may even allow hostile state actors to sabotage electric grids or other infrastructure by e-mailing employees malware disguised as medical notices. According to the consulting agency Accenture, data theft will cost the healthcare industry $305 billion between 2015 and 2019, with annual totals doubling from $40 billion to $80 billion.
Blockchain could put patients in control of their own data, empowering them to access, share, and even sell their medical information as they see fit.
One possible solution to this crisis involves radically retooling the way healthcare data is stored and shared—by using blockchain, the still-emerging information technology that underlies cryptocurrencies such as Bitcoin. And blockchain-enabled IT systems, boosters say, could do much more than prevent the theft of medical data. Such networks could revolutionize healthcare delivery on many levels, creating efficiencies that would reduce medical errors, improve coordination between providers, drive down costs, and give researchers unprecedented insights into patterns of disease. Perhaps most transformative, blockchain could put patients in control of their own data, empowering them to access, share, and even sell their medical information as they see fit. Widespread adoption could result in "a new kind of healthcare economy, in which data and services are quantifiable and exchangeable, with strong guarantees around both the security and privacy of sensitive information," wrote W. Brian Smith, chief scientist of healthcare-blockchain startup PokitDok, in a recent white paper.
Around the world, entrepreneurs, corporations, and government agencies are hopping aboard the blockchain train. A survey by the IBM Institute for Business Value, released in late 2016, found that 16 percent of healthcare executives in 16 countries planned to begin implementing some form of the technology in the coming year; 90 percent planned to launch a pilot program in the next two years. In 2017, Estonia became the first country to switch its medical-records system to a blockchain-based framework. Great Britain and Dubai are exploring a similar move. Yet in countries with more fragmented health systems, most notably the U.S., the challenges remain formidable. Some of the most advanced healthcare applications envisioned for blockchain, moreover, raise technological and ethical questions whose answers may not arrive anytime soon.
By creating a detailed, comprehensive, and immutable timeline of medical transactions, blockchain-based recordkeeping could help providers gauge a patient's long-term health patterns in a way that's never before been possible.
What Exactly Is Blockchain, Anyway?
To understand the buzz around blockchain, it's necessary to grasp (at least loosely) how the technology works. Ordinary digital recordkeeping systems rely on a central administrator that acts as gatekeeper to a treasury of data; if you can sneak past the guard, you can often gain access to the entire hoard, and your intrusion may go undetected indefinitely. Blockchain, by contrast, employs a network of synchronized, replicated databases. Information is scattered among these nodes, rather than on a single server, and is exchanged through encrypted, peer-to-peer pathways. Each transaction is visible to every computer on the network, and must be approved by a majority in order to be successfully completed. Each batch of transactions, or "block," is date- and time-stamped, marked with the user's identity, and given a cryptographic code, which is posted to every node. These blocks form a "chain," preserved in an electronic ledger, that can be read by all users but can't be edited. Any unauthorized access, or attempt at tampering, can be quickly neutralized by these overlapping safeguards. Even if a hacker managed to break into the system, penetrating deeply would be extraordinarily difficult.
Because blockchain technology shares transaction records throughout a network, it could eliminate communication bottlenecks between different components of the healthcare system (primary care physicians, specialists, nurses, and so on). And because blockchain-based systems are designed to incorporate programs known as "smart contracts," which automate functions previously requiring human intervention, they could reduce dangerous slipups as well as tedious and costly paperwork. For example, when a patient gets a checkup, sees a specialist, and fills a prescription, all these actions could be automatically recorded on his or her electronic health record (EHR), checked for errors, submitted for billing, and entered on insurance claims—which could be adjudicated and reimbursed automatically as well. "Blockchain has the potential to remove a lot of intermediaries from existing workflows, whether digital or nondigital," says Kamaljit Behera, an industry analyst for the consulting firm Frost & Sullivan.
The possible upsides don't end there. By creating a detailed, comprehensive, and immutable timeline of medical transactions, blockchain-based recordkeeping could help providers gauge a patient's long-term health patterns in a way that's never before been possible. In addition to data entered by their caregivers, individuals could use app-based technologies or wearables to transmit other information to their records, such as diet, exercise, and sleep patterns, adding new depth to their medical portraits.
Many experts expect healthcare blockchain to take root more slowly in the U.S. than in nations with government-run national health services.
Smart contracts could also allow patients to specify who has access to their data. "If you get an MRI and want your orthopedist to see it, you can add him to your network instead of carrying a CD into his office," explains Andrew Lippman, associate director of the MIT Media Lab, who helped create a prototype healthcare blockchain system called MedRec that's currently being tested at Beth Israel Deaconess Hospital in Boston. "Or you might make a smart contract to allow your son or daughter to access your healthcare records if something happens to you." Another option: permitting researchers to analyze your data for scientific purposes, whether anonymously or with your name attached.
The Recent History, and Looking Ahead
Over the past two years, a crowd of startups has begun vying for a piece of the emerging healthcare blockchain market. Some, like PokitDok and Atlanta-based Patientory, plan to mint proprietary cryptocurrencies, which investors can buy in lieu of stock, medical providers may earn as a reward for achieving better outcomes, and patients might score for meeting wellness goals or participating in clinical trials. (Patientory's initial coin offering, or ICO, raised more than $7 million in three days.) Several fledgling healthcare-blockchain companies have found powerful corporate partners: Intel for Silicon Valley's PokitDok, Kaiser Permanente for Patientory, Philips for Los Angeles-based Gem Health. At least one established provider network, Change Healthcare, is developing blockchain-based systems of its own. Two months ago, Change launched what it calls the first "enterprise-scale" blockchain network in U.S. healthcare—a system to track insurance claim submissions and remittances.
No one, however, has set a roll-out date for a full-blown, blockchain-based EHR system in this country. "We have yet to see anything move from the pilot phase to some kind of production status," says Debbie Bucci, an IT architect in the federal government's Office of the National Coordinator for Health Information Technology. Indeed, many experts expect healthcare blockchain to take root more slowly here than in nations with government-run national health services. In America, a typical patient may have dealings with a family doctor who keeps everything on paper, an assortment of hospitals that use different EHR systems, and an insurer whose system for processing claims is separate from that of the healthcare providers. To help bridge these gaps, a consortium called the Hyperledger Healthcare Working Group (which includes many of the leading players in the field) is developing standard protocols for blockchain interoperability and other functions. Adding to the complexity is the federal Health Insurance and Portability Act (HIPAA), which governs who can access patient data and under what circumstances. "Healthcare blockchain is in a very nascent stage," says Behera. "Coming up with regulations and other guidelines, and achieving large-scale implementation, will take some time."
The ethical implications of buying and selling personal genomic data in an electronic marketplace are doubtless open to debate.
How long? Behera, like other analysts, estimates that relatively simple applications, such as revenue-cycle management systems, could become commonplace in the next five years. More ambitious efforts might reach fruition in a decade or so. But once the infrastructure for healthcare blockchain is fully established, its uses could go far beyond keeping better EHRs.
A handful of scientists and entrepreneurs are already working to develop one visionary application: managing genomic data. Last month, Harvard University geneticist George Church—one of the most influential figures in his discipline—launched a business called Nebula Genomics. It aims to set up an exchange in which individuals can use "Neptune tokens" to purchase DNA sequencing, which will be stored in the company's blockchain-based system; research groups will be able to pay clients for their data using the same cryptocurrency. Luna DNA, founded by a team of biotech veterans in San Diego, plans a similar service, as does a Moscow-based startup called the Zenome Project.
Hossein Rahnama, CEO of the mobile-tech company Flybits and director of research at the Ryerson Centre for Cloud and Context-Aware Computing in Toronto, envisions a more personalized way of sharing genomic data via blockchain. His firm is working with a U.S. insurance company to develop a service that would allow clients in their 20s and 30s to connect with people in their 70s or 80s with similar genomes. The young clients would learn how the elders' lifestyle choices had influenced their health, so that they could modify their own habits accordingly. "It's intergenerational wisdom-sharing," explains Rahnama, who is 38. "I would actually pay to be a part of that network."
The ethical implications of buying and selling personal genomic data in an electronic marketplace are doubtless open to debate. Such commerce could greatly expand the pool of subjects for research in many areas of medicine, enabling the kinds of breakthroughs that only Big Data can provide. Yet it could also lead millions to surrender the most private information of all—the secrets of their cells—to buyers with less benign intentions. The Dark Overlord, one might argue, could not hope for a more satisfying victory.
These scenarios, however, are pure conjecture. After the first web page was posted, in 1991, Lippman observes, "a whole universe developed that you couldn't have imagined on Day 1." The same, he adds, is likely true for healthcare blockchain. "Our vision is to make medical records useful for you and for society, and to give you more control over your own identity. Time will tell."
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
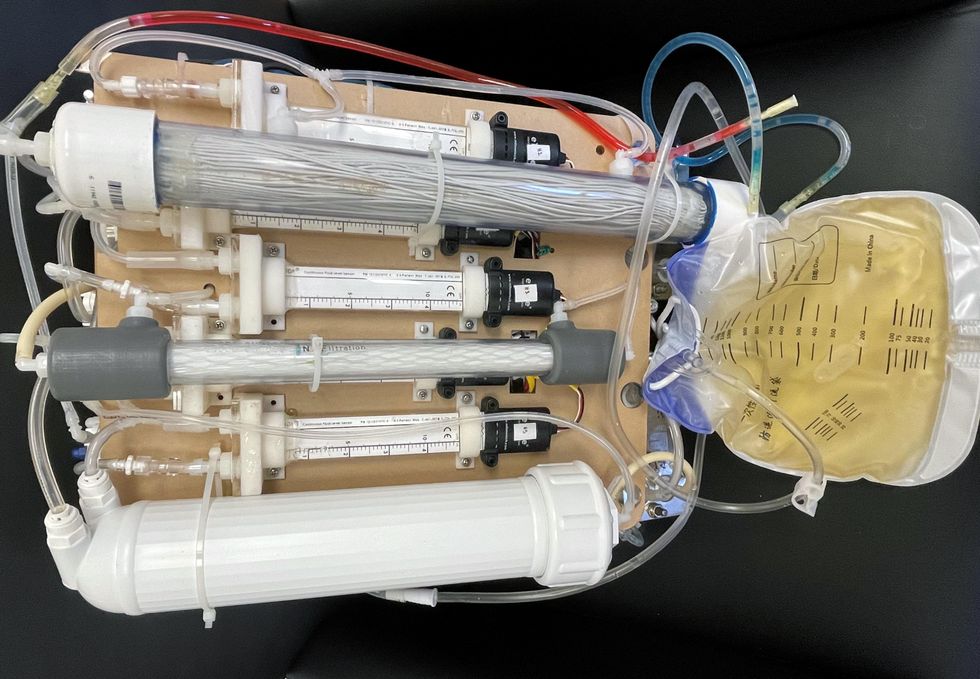
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
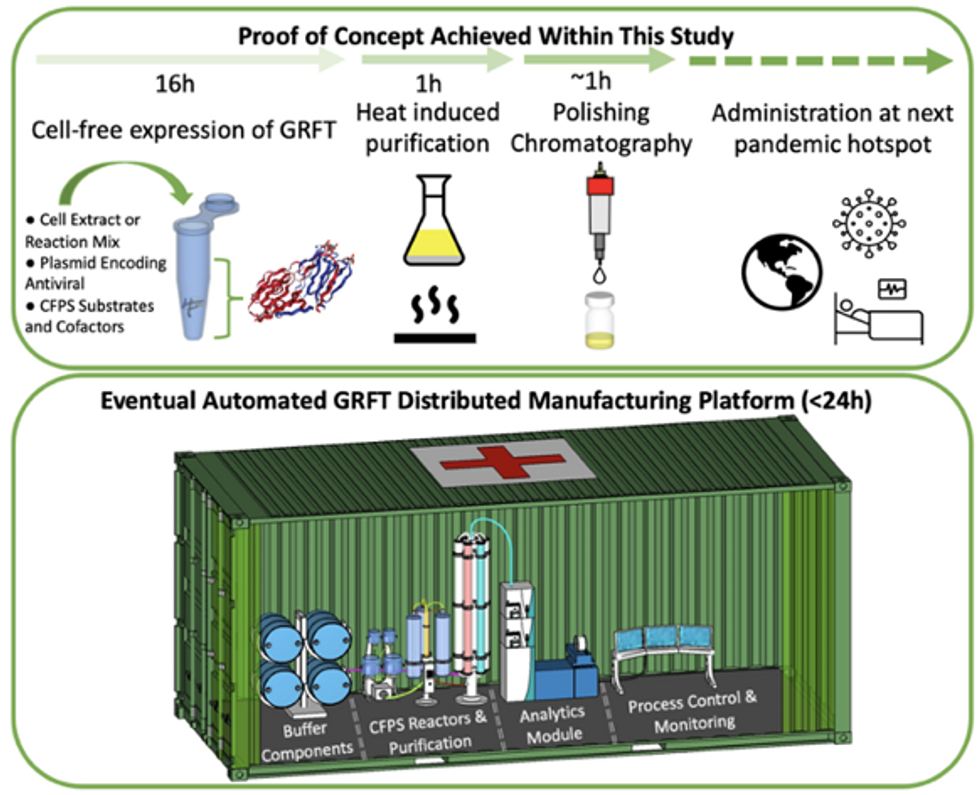
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.