World’s First “Augmented Reality” Contact Lens Aims to Revolutionize Much More Than Medicine
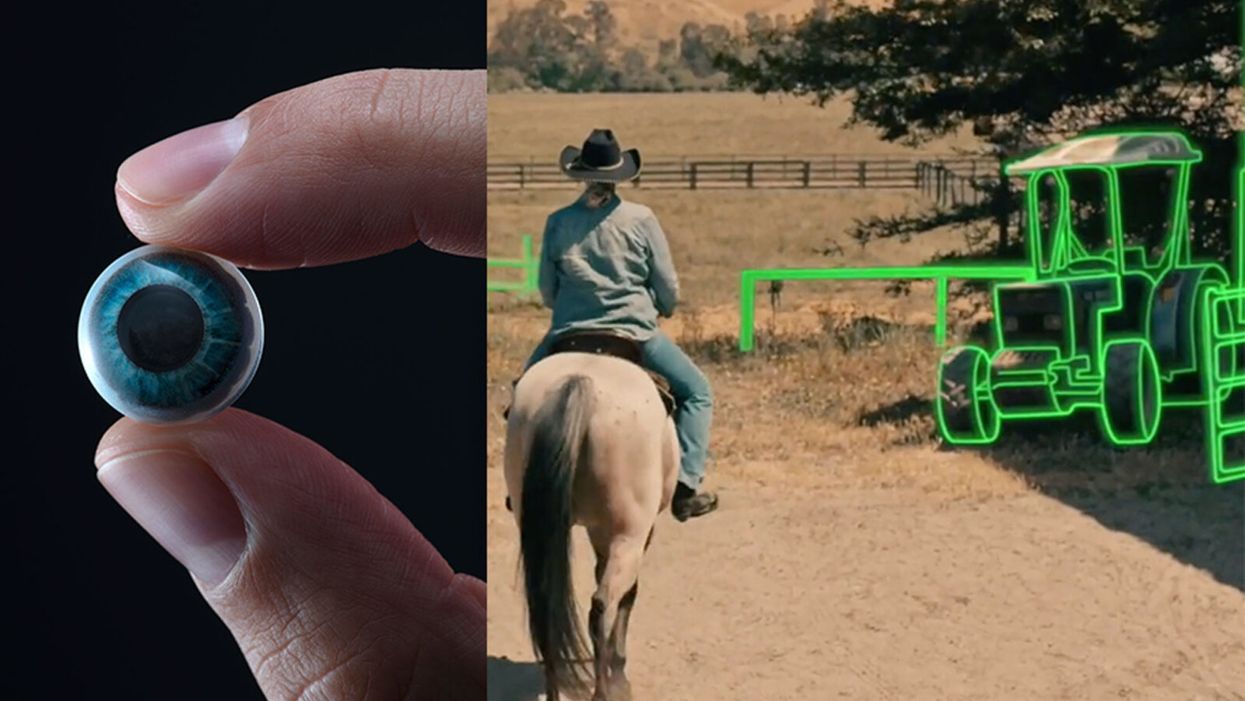
On the left, a picture of the Mojo lens smart contact; and a simulated image of a woman with low vision who is wearing the contact to highlight objects in her field of vision.
Imagine a world without screens. Instead of endlessly staring at your computer or craning your neck down to scroll through social media feeds and emails, information simply appears in front of your eyes when you need it and disappears when you don't.
"The vision is super clear...I was reading the poem with my eyes closed."
No more rude interruptions during dinner, no more bumping into people on the street while trying to follow GPS directions — just the information you want, when you need it, projected directly onto your visual field.
While this screenless future sounds like science fiction, it may soon be a reality thanks to the new Silicon Valley startup Mojo Vision, creator of the world's first smart contact lens. With a 14,000 pixel-per-inch display with eye-tracking, image stabilization, and a custom wireless radio, the Mojo smart lens bills itself the "smallest and densest dynamic display ever made." Unlike current augmented reality wearables such as Google Glass or ThirdEye, which project images onto a glass screen, the Mojo smart lens can project images directly onto the retina.
A current prototype displayed at the Consumer Electronics Show earlier this year in Las Vegas includes a tiny screen positioned right above the most sensitive area of the pupil. "[The Mojo lens] is a contact lens that essentially has wireless power and data transmission for a small micro LED projector that is placed over the center of the eye," explains David Hobbs, Director of Product Management at Mojo Vision. "[It] displays critical heads-up information when you need it and fades into the background when you're ready to continue on with your day."
Eventually, Mojo Visions' technology could replace our beloved smart devices but the first generation of the Mojo smart lens will be used to help the 2.2 billion people globally who suffer from vision impairment.
"If you think of the eye as a camera [for the visually impaired], the sensors are not working properly," explains Dr. Ashley Tuan, Vice President of Medical Devices at Mojo Vision and fellow of the American Academy of Optometry. "For this population, our lens can process the image so the contrast can be enhanced, we can make the image larger, magnify it so that low-vision people can see it or we can make it smaller so they can check their environment." In January of this year, the FDA granted Breakthrough Device Designation to Mojo, allowing them to have early and frequent discussions with the FDA about technical, safety and efficacy topics before clinical trials can be done and certification granted.
For now, Dr. Tuan is one of the few people who has actually worn the Mojo lens. "I put the contact lens on my eye. It was very comfortable like any contact lenses I've worn before," she describes. "The vision is super clear and then when I put on the accessories, suddenly I see Yoda in front of me and I see my vital signs. And then I have my colleague that prepared a beautiful poem that I loved when I was young [and] I was reading the poem with my eyes closed."
At the moment, there are several electronic glasses on the market like Acesight and Nueyes Pro that provide similar solutions for those suffering from visual impairment, but they are large, cumbersome, and highly visible. Mojo lens would be a discreet, more comfortable alternative that offers users more freedom of movement and independence.
"In the case of augmented-reality contact lenses, there could be an opportunity to improve the lives of people with low vision," says Dr. Thomas Steinemann, spokesperson for the American Academy of Ophthalmology and professor of ophthalmology at MetroHealth Medical Center in Cleveland. "There are existing tools for people currently living with low vision—such as digital apps, magnifiers, etc.— but something wearable could provide more flexibility and significantly more aid in day-to-day tasks."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless.
According to Dr. Tuan, the visually impaired often suffer from depression due to their lack of mobility and 70 percent of them are underemployed. "We hope that they can use this device to gain their mobility so they can get that social aspect back in their lives and then, eventually, employment," she explains. "That is our first and most important goal."
But helping those with low visual capabilities is only Mojo lens' first possible medical application; augmented reality is already being used in medicine and is poised to revolutionize the field in the coming decades. For example, Accuvein, a device that uses lasers to provide real-time images of veins, is widely used by nurses and doctors to help with the insertion of needles for IVs and blood tests.
According to the National Center for Biotechnology Information, augmentation of reality has been used in surgery for many years with surgeons using devices such as Google Glass to overlay critical information about their patients into their visual field. Using software like the Holographic Navigation Platform by Scopsis, surgeons can see a mixed-reality overlay that can "show you complicated tumor boundaries, assist with implant placements and guide you along anatomical pathways," its developers say.
However, according to Dr. Tuan, augmented reality headsets have drawbacks in the surgical setting. "The advantage of [Mojo lens] is you don't need to worry about sweating or that the headset or glasses will slide down to your nose," she explains "Also, our lens is designed so that it will understand your intent, so when you don't want the image overlay it will disappear, it will not block your visual field, and when you need it, it will come back at the right time."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless. Possibilities include live translation of sign language for deaf people; helping those with autism to read emotions; and improving doctors' bedside manner by allowing them to fully engage with patients without relying on a computer.
"[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
Furthermore, the lens could be used to monitor health issues. "We have image sensors in the lens right now that point to the world but we can have a camera pointing inside of your eye to your retina," says Dr. Tuan, "[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
For the moment, the future medical applications of the Mojo lens are still theoretical, but the team is confident they can eventually become a reality after going through the proper regulatory review. The company is still in the process of design, prototype and testing of the lens, so they don't know exactly when it will be available for use, but they anticipate shipping the first available products in the next couple of years. Once it does go to market, it will be available by prescription only for those with visual impairments, but the team's goal is to bring it to broader consumer markets pending regulatory clearance.
"We see that right now there's a unique opportunity here for Mojo lens and invisible computing to help to shape what the next decade of technology development looks like," explains David Hobbs. "We can use [the Mojo lens] to better serve us as opposed to us serving technology better."
In this week's Friday Five, new research that could help prevent Alzheimer's. Plus, why you should care about smart senior towns, how to reverse being drunk, money can make you happier if you're this type of person, and personalized anxiety medicine.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. Christopher Martens, director of the Delaware Center for Cogntiive Aging Research and professor of kinesiology and applied physiology at the University of Delaware, and Dr. Ilona Matysiak, visiting scholar at Iowa State University and associate professor of sociology at Maria Grzegorzewska University.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
- Could this supplement help prevent Alzheimer's?
- Why you should care about smart senior towns
- Here's how to reverse being drunk
- Money can make you happy - if you're this type of person
- Personalized anxiety medicine
As gene therapies and small molecule drugs are being studied in clinical trials, companies increasingly see the value in hiring patients to help explain the potential benefits.
As a child, Wendy Borsari participated in a health study at Boston Children’s Hospital. She was involved because heart disease and sudden cardiac arrest ran in her family as far back as seven generations. When she was 18, however, the study’s doctors told her that she had a perfectly healthy heart and didn’t have to worry.
A couple of years after graduating from college, though, the Boston native began to experience episodes of near fainting. During any sort of strenuous exercise, my blood pressure would drop instead of increasing, she recalls.
She was diagnosed at 24 with hypertrophic cardiomyopathy. Although HCM is a commonly inherited heart disease, Borsari’s case resulted from a rare gene mutation, the MYH7 gene. Her mother had been diagnosed at 27, and Borsari had already lost her grandmother and two maternal uncles to the condition. After her own diagnosis, Borsari spent most of her free time researching the disease and “figuring out how to have this condition and still be the person I wanted to be,” she says.
Then, her son was found to have the genetic mutation at birth and diagnosed with HCM at 15. Her daughter, also diagnosed at birth, later suffered five cardiac arrests.
That changed Borsari’s perspective. She decided to become a patient advocate. “I didn’t want to just be a patient with the condition,” she says. “I wanted to be more involved with the science and the biopharmaceutical industry so I could be active in helping to make it better for other patients.”
She consulted on patient advocacy for a pharmaceutical and two foundations before coming to a company called Tenaya in 2021.
“One of our core values as a company is putting patients first,” says Tenaya's CEO, Faraz Ali. “We thought of no better way to put our money where our mouth is than by bringing in somebody who is affected and whose family is affected by a genetic form of cardiomyopathy to have them make sure we’re incorporating the voice of the patient.”
Biomedical corporations and government research agencies are now incorporating patient advocacy more than ever, says Alice Lara, president and CEO of the Sudden Arrhythmia Death Syndromes Foundation in Salt Lake City, Utah. These organizations have seen the effectiveness of including patient voices to communicate and exemplify the benefits that key academic research institutions have shown in their medical studies.
“From our side of the aisle,” Lara says, “what we know as patient advocacy organizations is that educated patients do a lot better. They have a better course in their therapy and their condition, and understanding the genetics is important because all of our conditions are genetic.”
Founded in 2016, Tenaya is advancing gene therapies and small molecule drugs in clinical trials for both prevalent and rare forms of heart disease, says Ali, the CEO.
The firm's first small molecule, now in a Phase 1 clinical trial, is intended to treat heart failure with preserved ejection fraction, where the amount of blood pumped by the heart is reduced due to the heart chambers becoming weak or stiff. The condition accounts for half or more of all heart failure in the U.S., according to Ali, and is growing quickly because it's closely associated with diabetes. It’s also linked with metabolic syndrome, or a cluster of conditions including high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels.
“We have a novel molecule that is first in class and, to our knowledge, best in class to tackle that, so we’re very excited about the clinical trial,” Ali says.
The first phase of the trial is being performed with healthy participants, rather than people with the disease, to establish safety and tolerability. The researchers can also look for the drug in blood samples, which could tell them whether it's reaching its target. Ali estimates that, if the company can establish safety and that it engages the right parts of the body, it will likely begin dosing patients with the disease in 2024.
Tenaya’s therapy delivers a healthy copy of the gene so that it makes a copy of the protein missing from the patients' hearts because of their mutation. The study will start with adult patients, then pivot potentially to children and even newborns, Ali says, “where there is an even greater unmet need because the disease progresses so fast that they have no options.”
Although this work still has a long way to go, Ali is excited about the potential because the gene therapy achieved positive results in the preclinical mouse trial. This animal trial demonstrated that the treatment reduced enlarged hearts, reversed electrophysiological abnormalities, and improved the functioning of the heart by increasing the ejection fraction after the single-dose of gene therapy. That measurement remained stable to the end of the animals’ lives, roughly 18 months, Ali says.
He’s also energized by the fact that heart disease has “taken a page out of the oncology playbook” by leveraging genetic research to develop more precise and targeted drugs and gene therapies.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” says Melind Desai of the Cleveland Clinic.
Tenaya’s second program focuses on developing a gene therapy to mitigate the leading cause of hypertrophic cardiomyopathy through a specific gene called MYPBC3. The disease affects approximately 600,000 patients in the U.S. This particular genetic form, Ali explains, affects about 115,000 in the U.S. alone, so it is considered a rare disease.
“There are infants who are dying within the first weeks to months of life as a result of this mutation,” he says. “There are also adults who start having symptoms in their 20s, 30s and 40s with early morbidity and mortality.” Tenaya plans to apply before the end of this year to get the FDA’s approval to administer an investigational drug for this disease humans. If approved, the company will begin to dose patients in 2023.
“We now understand the genetics of the heart much better,” he says. “We now understand the leading genetic causes of hypertrophic myopathy, dilated cardiomyopathy and others, so that gives us the ability to take these large populations and stratify them rationally into subpopulations.”
Melind Desai, MD, who directs Cleveland Clinic’s Hypertrophic Cardiomyopathy Center, says that the goal of Tenaya’s second clinical study is to help improve the basic cardiac structure in patients with hypertrophic cardiomyopathy related to the MYPBC3 mutation.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” he says. “So this is an exciting new frontier of therapeutic investigation for MYPBC3 gene-positive patients with a chance for a cure.
Neither of Tenaya’s two therapies address the gene mutation that has affected Borsari and her family. But Ali sees opportunity down the road to develop a gene therapy for her particular gene mutation, since it is the second leading cause of cardiomyopathy. Treating the MYH7 gene is especially challenging because it requires gene editing or silencing, instead of just replacing the gene.

Wendy Borsari was diagnosed at age 24 with a commonly inherited heart disease. She joined Tenaya as a patient advocate in 2021.
Wendy Borsari
“If you add a healthy gene it will produce healthy copies,” Ali explains, “but it won’t stop the bad effects of the mutant protein the gene produces. You can only do that by silencing the gene or editing it out, which is a different, more complicated approach.”
Euan Ashley, professor of medicine and genetics at Stanford University and founding director of its Center for Inherited Cardiovascular Disease, is confident that we will see genetic therapies for heart disease within the next decade.
“We are at this really exciting moment in time where we have diseases that have been under-recognized and undervalued now being attacked by multiple companies with really modern tools,” says Ashley, author of The Genome Odyssey. “Gene therapies are unusual in the sense that they can reverse the cause of the disease, so we have the enticing possibility of actually reversing or maybe even curing these diseases.”
Although no one is doing extensive research into a gene therapy for her particular mutation yet, Borsari remains hopeful, knowing that companies such as Tenaya are moving in that direction.
“I know that’s now on the horizon,” she says. “It’s not just some pipe dream, but will happen hopefully in my lifetime or my kids’ lifetime to help them.”

