Your Genetic Data Is The New Oil. These Startups Will Pay to Rent It.

A doctor conducts a test on DNA.
Perhaps you're one of the 12 million people who has spit into a tube in recent years to learn the secrets in your genetic code, like your ancestry, your health risks, or your carrier status for certain diseases. If you haven't participated in direct-to-consumer genetic testing, you may know someone who has.
It's for people who want more control over their genetic data--plus a share of the proceeds when and if that data is used.
Mountains of genomic data have been piling up steeply over the last several years, but according to some experts, not enough research and drug discovery is being done with the data collected, and customers rarely have a say in how their data is used. Now, a slew of ambitious startup companies are bringing together the best of blockchain technology and human genomics to help solve these problems.
But First, Why Is Your Genome So Valuable?
Access to genetic information is an obvious boon to scientific and medical progress. In the right hands, it has the potential to save lives and reduce suffering — by facilitating the development of better, safer, more targeted treatments and by shedding light on the role of genetics in countless diseases and medical conditions.
Research requiring access to direct-to-consumer (DTC) genomic data is already well underway. For example, 23andMe, the popular California-based DTC genetic testing company, has published 107 research articles so far, as of this May, using data from their five million-plus customers around the world. Their website states that, on average, of the 80 percent of their customers who have opted to share their genomic data for research purposes, each "individual contributes to 200 different research studies."
And this July, a new collaboration was announced between 23andMe and GlaxoSmithKline, the London-based pharmaceutical company. GlaxoSmithKline will be using data from 23andMe customers to develop new medical treatments, while 23andMe will receive $300 million from the four-year deal. Both companies are poised to profit significantly from their union.
Should 23andMe's customers share in the gains? Peter Pitts, president of the Center for Medicine in the Public Interest, believes they should. "Are they going to offer rebates to people who opt in, so their customers aren't paying for the privilege of 23andMe working with a for-profit company in a for-profit research project?" Pitts told NBC. So far, 23andMe has not announced any plans to share profits with their customers.
But outside of such major partnerships, many researchers are frustrated by the missed opportunities to dig deeper into the correlations between genetics and disease. That's because people's de-identified genomic information is "essentially lying fallow," siloed behind significant security blockades in the interest of preserving their anonymity. So how can both researchers and consumers come out ahead?
Putting Consumers Back in Control
For people who want more control over their genetic data -- plus a share of the proceeds when and if that data is used -- a few companies have paired consumer genomics with blockchain technology to form a new field called "blockchain genomics." Blockchain is a data storage technology that relies on a network of computers, or peer-to-peer setup, making it incredibly difficult to hack. "It's a closed loop of transactions that gets protected and encrypted, and it cannot be changed," says Tanya Woods, a blockchain thought leader and founder of Kind Village, a social impact technology platform.
The vision is to incentivize consumers to share their genomic data and empower researchers to make new breakthroughs.
"So if I agree to give you something and you agree to accept it, we make that exchange, and then that basic framework is captured in a block. … Anything that can be exchanged can be ledgered on blockchain. Anything. It could be real estate, it could be the transfer of artwork, it could be the purchase of a song or any digital content, it could be recognition of a certification," and so on.
The blockchain genomics companies' vision is to incentivize consumers to share their genomic data and empower researchers to make new breakthroughs, all while keeping the data secure and the identities of consumers anonymous.
Consumers, or "partners" as these companies call them, will have a direct say regarding which individuals or organizations can "rent" their data, and will be able to negotiate the amount they receive in exchange. But instead of fiat currency (aka "regular money") as payment, partners will either be remunerated in cryptocurrency unique to the specific company or they will be provided with individual shares of ownership in the database for contributing DNA data and other medical information.
Luna DNA, one of the blockchain genomics companies, "will allow any credible researcher or non-profit to access the databases for a nominal fee," says its president and co-founder, Dawn Barry. Luna DNA's infrastructure was designed to embrace certain conceptions of privacy and privacy law "in which individuals are in total control of their data, including the ability to have their data be 'forgotten' at any time," she said. This is nearly impossible to implement in pre-existing systems that were not designed with full control by the individual in mind.
One of the legal instruments to which Barry referred was the European Union's General Data Protection Regulation, which "states that the data collected on an individual is owned and should be controlled by that individual," she explained. Another is the California Privacy Act that echoes similar principles. "There is a global trend towards more control by the individual that has very deep implications to companies and sites that collect and aggregate data."
David Koepsell, CEO and co-founder of EncrypGen, told Forbes that "Most people are not aware that your DNA contains information about your life expectancy, your proclivity to depression or schizophrenia, your complete ethnic ancestry, your expected intelligence, maybe even your political inclinations" — information that could be misused by insurance companies and employers. And though DTC customers have been assured that their data will stay anonymous, some data can be linked back to consumers' identities. Blockchain may be the answer to these concerns.
Both blockchain technology and the DTC genetic testing arena have a glaring diversity problem.
"The security that's provided by blockchain is tremendous," Woods says. "It's a significant improvement … and as we move toward more digitized economies around the world, these kinds of solutions that are providing security, validity, trust — they're very important."
In the case of blockchain genomics companies like EncrypGen, Luna DNA, Longenesis, and Zenome, each partner who joins would bring a digital copy of their genetic readout from DTC testing companies (like 23andMe or AncestryDNA). The blockchain technology would then be used to record how and for what purposes researchers interact with it. (To learn more about blockchain, check out this helpful visual guide by Reuters.)
Obstacles in the Path to Success
The cryptocurrency approach as a method of payment could be an unattractive lure to consumers if only a limited number of people make transactions in a given currency's network. And the decade-old technology underlying it -- blockchain -- is not yet widely supported, or even well-understood, by the public at large.
"People conflate blockchain with cryptocurrency and bitcoin and all of the concerns and uncertainty thereof," Barry told us. "One can think of cryptocurrency as a single expression of the vast possibilities of the blockchain technology. Blockchain is straightforward in concept and arcane in its implementation."
But blockchain, with its Gini coefficient of 0.98, is one of the most unequal "playing fields" around. The Gini coefficient is a measure of economic inequality, where 0 represents perfect equality and 1 represents perfect inequality. Around 90 percent of bitcoin users, for example, are male, white or Asian, between the ages of 18 and 34, straight, and from middle and upper class families.
The DTC genetic testing arena, too, has a glaring diversity problem. Most DTC genetic test consumers, just like most genetic study participants, are of European descent. In the case of genetic studies, this disparity is largely explained by the fact that most research is done in Europe and North America. In addition to being over 85 percent white, individuals who purchase DTC genetic testing kits are highly educated (about half have more than a college degree), well off (43 percent have a household income of $100,000 or more per year), and are politically liberal (almost 65 percent). Only 14.5 percent of DTC genetic test consumers are non-white, and a mere 5 percent are Hispanic.
Since risk of genetic diseases often varies greatly between ethnic groups, results from DTC tests can be less accurate and less specific for those of non-European ancestry — simply due to a lack of diverse data. The bigger the genetic database, wrote Sarah Zhang for The Atlantic, the more insights 23andMe and other DTC companies "can glean from DNA. That, in turn, means the more [they] can tell customers about their ancestry and health…" Though efforts at recruiting non-white participants have been ongoing, and some successes have been made at improving ancestry tools for people of color, the benefits of genomic gathering in North America are still largely reaped by Caucasians.
So far, it's not yet clear who or how many people will choose to partake in the offerings of blockchain genomics companies.
So one chief hurdle for the blockchain genomics companies is getting the technology into the hands of those who are under-represented in both blockchain and genetic testing research. Women, in particular, may be difficult to bring on board the blockchain genomics bandwagon — though not from lack of interest. Although women make up a significant portion of DTC genetic testing customers (between 50 and 60 percent), their presence is lacking in blockchain and the biotech industry in general.
At the North American Bitcoin Conference in Miami earlier this year, only three women were on stage, compared to 84 men. And the after-party was held in a strip club.
"I was at that conference," Woods told us. "I don't know what happened at the strip club, I didn't observe it. That's not to say it didn't happen … but I enjoyed being at the conference and I enjoyed learning from people who are experimenting in the space and developing in it. Generally, would I have loved to see more women visible? Of course. In tech generally I want to see more women visible, but there's a whole ecosystem shifting that has to happen to make that possible."
Luna's goal is to achieve equal access to a technology (blockchain genomics) that could potentially improve health and quality of life for all involved. But in the merging of two fields that have been unequal since their inception, achieving equal access is one tall order indeed. So far, it's not yet clear who or how many people will choose to participate. LunaDNA's platform has not yet launched; EncrypGen released their beta version just last month.
Sharon Terry, president and CEO of Genetic Alliance — a nonprofit organization that advocates for access to quality genetic services — recently shared a message that reflects the zeitgeist for all those entering the blockchain genomics space: "Be authentic. Tell the truth, even about motives and profits. Be transparent. Engage us. Don't leave us out. Make this real collaboration. Be bold. Take risks. People are dying. It's time to march forward and make a difference."
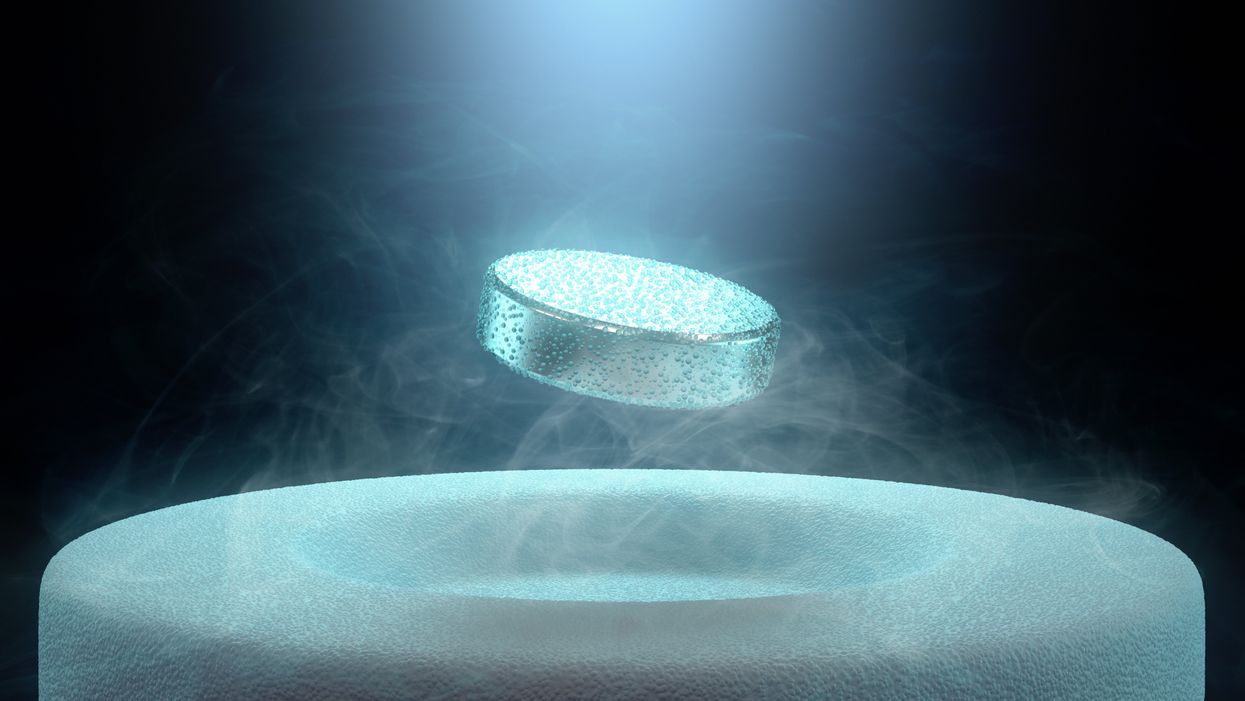
Researchers claimed they built a breakthrough superconductor. Social media shot it down almost instantly.
In July, South Korean scientists posted a paper finding they had achieved superconductivity - a claim that was debunked within days.
Harsh Mathur was a graduate physics student at Yale University in late 1989 when faculty announced they had failed to replicate claims made by scientists at the University of Utah and the University of Wolverhampton in England.
Such work is routine. Replicating or attempting to replicate the contraptions, calculations and conclusions crafted by colleagues is foundational to the scientific method. But in this instance, Yale’s findings were reported globally.
“I had a ringside view, and it was crazy,” recalls Mathur, now a professor of physics at Case Western Reserve University in Ohio.
Yale’s findings drew so much attention because initial experiments by Stanley Pons of Utah and Martin Fleischmann of Wolverhampton led to a startling claim: They were able to fuse atoms at room temperature – a scientific El Dorado known as “cold fusion.”
Nuclear fusion powers the stars in the universe. However, star cores must be at least 23.4 million degrees Fahrenheit and under extraordinary pressure to achieve fusion. Pons and Fleischmann claimed they had created an almost limitless source of power achievable at any temperature.
Like fusion, superconductivity can only be achieved in mostly impractical circumstances.
But about six months after they made their startling announcement, the pair’s findings were discredited by researchers at Yale and the California Institute of Technology. It was one of the first instances of a major scientific debunking covered by mass media.
Some scholars say the media attention for cold fusion stemmed partly from a dazzling announcement made three years prior in 1986: Scientists had created the first “superconductor” – material that could transmit electrical current with little or no resistance. It drew global headlines – and whetted the public’s appetite for announcements of scientific breakthroughs that could cause economic transformations.
But like fusion, superconductivity can only be achieved in mostly impractical circumstances: It must operate either at temperatures of at least negative 100 degrees Fahrenheit, or under pressures of around 150,000 pounds per square inch. Superconductivity that functions in closer to a normal environment would cut energy costs dramatically while also opening infinite possibilities for computing, space travel and other applications.
In July, a group of South Korean scientists posted material claiming they had created an iron crystalline substance called LK-99 that could achieve superconductivity at slightly above room temperature and at ambient pressure. The group partners with the Quantum Energy Research Centre, a privately-held enterprise in Seoul, and their claims drew global headlines.
Their work was also debunked. But in the age of internet and social media, the process was compressed from half-a-year into days. And it did not require researchers at world-class universities.
One of the most compelling critiques came from Derrick VanGennep. Although he works in finance, he holds a Ph.D. in physics and held a postdoctoral position at Harvard. The South Korean researchers had posted a video of a nugget of LK-99 in what they claimed was the throes of the Meissner effect – an expulsion of the substance’s magnetic field that would cause it to levitate above a magnet. Unless Hollywood magic is involved, only superconducting material can hover in this manner.
That claim made VanGennep skeptical, particularly since LK-99’s levitation appeared unenthusiastic at best. In fact, a corner of the material still adhered to the magnet near its center. He thought the video demonstrated ferromagnetism – two magnets repulsing one another. He mixed powdered graphite with super glue, stuck iron filings to its surface and mimicked the behavior of LK-99 in his own video, which was posted alongside the researchers’ video.
VanGennep believes the boldness of the South Korean claim was what led to him and others in the scientific community questioning it so quickly.
“The swift replication attempts stemmed from the combination of the extreme claim, the fact that the synthesis for this material is very straightforward and fast, and the amount of attention that this story was getting on social media,” he says.
But practicing scientists were suspicious of the data as well. Michael Norman, director of the Argonne Quantum Institute at the Argonne National Laboratory just outside of Chicago, had doubts immediately.
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication.
“It wasn’t a very polished paper,” Norman says of the Korean scientists’ work. That opinion was reinforced, he adds, when it turned out the paper had been posted online by one of the researchers prior to seeking publication in a peer-reviewed journal. Although Norman and Mathur say that is routine with scientific research these days, Norman notes it was posted by one of the junior researchers over the doubts of two more senior scientists on the project.
Norman also raises doubts about the data reported. Among other issues, he observes that the samples created by the South Korean researchers contained traces of copper sulfide that could inadvertently amplify findings of conductivity.
The lack of the Meissner effect also caught Mathur’s attention. “Ferromagnets tend to be unstable when they levitate,” he says, adding that the video “just made me feel unconvinced. And it made me feel like they hadn't made a very good case for themselves.”
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication. Despite being debunked, cold fusion claimants Pons and Fleischmann didn’t disappear. They moved their research to automaker Toyota’s IMRA laboratory in France, which along with the Japanese government spent tens of millions of dollars on their work before finally pulling the plug in 1998.
Fusion has since been created in laboratories, but being unable to reproduce the density of a star’s core would require excruciatingly high temperatures to achieve – about 160 million degrees Fahrenheit. A recently released Government Accountability Office report concludes practical fusion likely remains at least decades away.
However, like Pons and Fleischman, the South Korean researchers are not going anywhere. They claim that LK-99’s Meissner effect is being obscured by the fact the substance is both ferromagnetic and diamagnetic. They have filed for a patent in their country. But for now, those claims remain chimerical.
In the meantime, the consensus as to when a room temperature superconductor will be achieved is mixed. VenGennep – who studied the issue during his graduate and postgraduate work – puts the chance of creating such a superconductor by 2050 at perhaps 50-50. Mathur believes it could happen sooner, but adds that research on the topic has been going on for nearly a century, and that it has seen many plateaus.
“There's always this possibility that there's going to be something out there that we're going to discover unexpectedly,” Norman notes. The only certainty in this age of social media is that it will be put through the rigors of replication instantly.
Scientists implant brain cells to counter Parkinson's disease
In a recent research trial, patients with Parkinson's disease reported that their symptoms had improved after stem cells were implanted into their brains. Martin Taylor, far right, was diagnosed at age 32.
Martin Taylor was only 32 when he was diagnosed with Parkinson's, a disease that causes tremors, stiff muscles and slow physical movement - symptoms that steadily get worse as time goes on.
“It's horrible having Parkinson's,” says Taylor, a data analyst, now 41. “It limits my ability to be the dad and husband that I want to be in many cruel and debilitating ways.”
Today, more than 10 million people worldwide live with Parkinson's. Most are diagnosed when they're considerably older than Taylor, after age 60. Although recent research has called into question certain aspects of the disease’s origins, Parkinson’s eventually kills the nerve cells in the brain that produce dopamine, a signaling chemical that carries messages around the body to control movement. Many patients have lost 60 to 80 percent of these cells by the time they are diagnosed.
For years, there's been little improvement in the standard treatment. Patients are typically given the drug levodopa, a chemical that's absorbed by the brain’s nerve cells, or neurons, and converted into dopamine. This drug addresses the symptoms but has no impact on the course of the disease as patients continue to lose dopamine producing neurons. Eventually, the treatment stops working effectively.
BlueRock Therapeutics, a cell therapy company based in Massachusetts, is taking a different approach by focusing on the use of stem cells, which can divide into and generate new specialized cells. The company makes the dopamine-producing cells that patients have lost and inserts these cells into patients' brains. “We have a disease with a high unmet need,” says Ahmed Enayetallah, the senior vice president and head of development at BlueRock. “We know [which] cells…are lost to the disease, and we can make them. So it really came together to use stem cells in Parkinson's.”
In a phase 1 research trial announced late last month, patients reported that their symptoms had improved after a year of treatment. Brain scans also showed an increased number of neurons generating dopamine in patients’ brains.
Increases in dopamine signals
The recent phase 1 trial focused on deploying BlueRock’s cell therapy, called bemdaneprocel, to treat 12 patients suffering from Parkinson’s. The team developed the new nerve cells and implanted them into specific locations on each side of the patient's brain through two small holes in the skull made by a neurosurgeon. “We implant cells into the places in the brain where we think they have the potential to reform the neural networks that are lost to Parkinson's disease,” Enayetallah says. The goal is to restore motor function to patients over the long-term.
Five patients were given a relatively low dose of cells while seven got higher doses. Specialized brain scans showed evidence that the transplanted cells had survived, increasing the overall number of dopamine producing cells. The team compared the baseline number of these cells before surgery to the levels one year later. “The scans tell us there is evidence of increased dopamine signals in the part of the brain affected by Parkinson's,” Enayetallah says. “Normally you’d expect the signal to go down in untreated Parkinson’s patients.”
"I think it has a real chance to reverse motor symptoms, essentially replacing a missing part," says Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh.
The team also asked patients to use a specific type of home diary to log the times when symptoms were well controlled and when they prevented normal activity. After a year of treatment, patients taking the higher dose reported symptoms were under control for an average of 2.16 hours per day above their baselines. At the smaller dose, these improvements were significantly lower, 0.72 hours per day. The higher-dose patients reported a corresponding decrease in the amount of time when symptoms were uncontrolled, by an average of 1.91 hours, compared to 0.75 hours for the lower dose. The trial was safe, and patients tolerated the year of immunosuppression needed to make sure their bodies could handle the foreign cells.
Claire Bale, the associate director of research at Parkinson's U.K., sees the promise of BlueRock's approach, while noting the need for more research on a possible placebo effect. The trial participants knew they were getting the active treatment, and placebo effects are known to be a potential factor in Parkinson’s research. Even so, “The results indicate that this therapy produces improvements in symptoms for Parkinson's, which is very encouraging,” Bale says.
Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh, also finds the results intriguing. “I think it's excellent,” he says. “I think it has a real chance to reverse motor symptoms, essentially replacing a missing part.” However, it could take time for this therapy to become widely available, Kunath says, and patients in the late stages of the disease may not benefit as much. “Data from cell transplantation with fetal tissue in the 1980s and 90s show that cells did not survive well and release dopamine in these [late-stage] patients.”
Searching for the right approach
There's a long history of using cell therapy as a treatment for Parkinson's. About four decades ago, scientists at the University of Lund in Sweden developed a method in which they transferred parts of fetal brain tissue to patients with Parkinson's so that their nerve cells would produce dopamine. Many benefited, and some were able to stop their medication. However, the use of fetal tissue was highly controversial at that time, and the tissues were difficult to obtain. Later trials in the U.S. showed that people benefited only if a significant amount of the tissue was used, and several patients experienced side effects. Eventually, the work lost momentum.
“Like many in the community, I'm aware of the long history of cell therapy,” says Taylor, the patient living with Parkinson's. “They've long had that cure over the horizon.”
In 2000, Lorenz Studer led a team at the Memorial Sloan Kettering Centre, in New York, to find the chemical signals needed to get stem cells to differentiate into cells that release dopamine. Back then, the team managed to make cells that produced some dopamine, but they led to only limited improvements in animals. About a decade later, in 2011, Studer and his team found the specific signals needed to guide embryonic cells to become the right kind of dopamine producing cells. Their experiments in mice, rats and monkeys showed that their implanted cells had a significant impact, restoring lost movement.
Studer then co-founded BlueRock Therapeutics in 2016. Forming the most effective stem cells has been one of the biggest challenges, says Enayetallah, the BlueRock VP. “It's taken a lot of effort and investment to manufacture and make the cells at the right scale under the right conditions.” The team is now using cells that were first isolated in 1998 at the University of Wisconsin, a major advantage because they’re available in a virtually unlimited supply.
Other efforts underway
In the past several years, University of Lund researchers have begun to collaborate with the University of Cambridge on a project to use embryonic stem cells, similar to BlueRock’s approach. They began clinical trials this year.
A company in Japan called Sumitomo is using a different strategy; instead of stem cells from embryos, they’re reprogramming adults' blood or skin cells into induced pluripotent stem cells - meaning they can turn into any cell type - and then directing them into dopamine producing neurons. Although Sumitomo started clinical trials earlier than BlueRock, they haven’t yet revealed any results.
“It's a rapidly evolving field,” says Emma Lane, a pharmacologist at the University of Cardiff who researches clinical interventions for Parkinson’s. “But BlueRock’s trial is the first full phase 1 trial to report such positive findings with stem cell based therapies.” The company’s upcoming phase 2 research will be critical to show how effectively the therapy can improve disease symptoms, she added.
The cure over the horizon
BlueRock will continue to look at data from patients in the phase 1 trial to monitor the treatment’s effects over a two-year period. Meanwhile, the team is planning the phase 2 trial with more participants, including a placebo group.
For patients with Parkinson’s like Martin Taylor, the therapy offers some hope, though Taylor recognizes that more research is needed.

BlueRock Therapeutics
“Like many in the community, I'm aware of the long history of cell therapy,” he says. “They've long had that cure over the horizon.” His expectations are somewhat guarded, he says, but, “it's certainly positive to see…movement in the field again.”
"If we can demonstrate what we’re seeing today in a more robust study, that would be great,” Enayetallah says. “At the end of the day, we want to address that unmet need in a field that's been waiting for a long time.”
Editor's note: The company featured in this piece, BlueRock Therapeutics, is a portfolio company of Leaps by Bayer, which is a sponsor of Leaps.org. BlueRock was acquired by Bayer Pharmaceuticals in 2019. Leaps by Bayer and other sponsors have never exerted influence over Leaps.org content or contributors.