23andMe Is Using Customers’ Genetic Data to Develop Drugs. Is This Brilliant or Dubious?

A woman does a DNA test with a cotton swab at home.
Leading direct-to-consumer (DTC) genetic testing companies are continuously unveiling novel ways to leverage their vast stores of genetic data.
"23andMe will tell you what diseases you have and then sell you the drugs to treat them."
As reported last week, 23andMe's latest concept is to develop and license new drugs using the data of consumers who have opted in to let their information be used for research. To date, over 10 million people have used the service and around 80 percent have opted in, making its database one of the largest in the world.
Culture researcher Dr. Julia Creet is one of the foremost experts on the DTC genetic testing industry, and in her forthcoming book, The Genealogical Sublime, she bluntly examines whether such companies' motives and interests are in sync with those of consumers.
Leapsmag caught up with Creet about the latest news and the wider industry's implications for health and privacy.
23andMe has just announced that it plans to license a newly developed anti-inflammatory drug, the first one created using its customers' genetic data, to Almirall, a pharma company in Spain. What's your take?
I think this development is the next step in the evolution of the company and its "double-sided" marketing model. In the past, as it enticed customers to give it their DNA, it sold the results and the medical information divulged by customers to other drug companies. Now it is positioning itself to reap the profits of a new model by developing treatments itself.
Given that there are many anti-inflammatory drugs on the market already, whatever Almirall produces might not have much of an impact. We might see this canny move as a "proof of concept," that 23andMe has learned how to "leverage" its genetic data without having to sell them to a third party. In a way, the privacy provisions will be much less complicated, and the company stands to attract investment as it turns itself into [a pseudo pharmaceutical company], a "pharma-psuedocal" company.
Emily Drabant Conley, the president of business development, has said that 23andMe is pursuing other drug compounds and may conduct their own clinical trials rather than licensing them out to their existing research partners. The end goal, it seems, is to make direct-to-consumer DNA testing to drug production and sales back to that same consumer base a seamless and lucrative circle. You have to admit it's a brilliant business model. 23andMe will tell you what diseases you have and then sell you the drugs to treat them.
In your new book, you describe how DTC genetic testing companies have capitalized on our innate human desire to connect with or ancestors and each other. I quote you: "This industry has taken that potent, spiritual, all-too-human need to belong... and monetized it in a particularly exploitative way." But others argue that DTC genetic testing companies are merely providing a service in exchange for fair-market compensation. So where does exploitation come into the picture?
Yes, the industry provides a fee for service, but that's only part of the story. The rest of the story reveals a pernicious industry that hides its business model behind the larger science project of health and heredity. All of the major testing companies play on the idea of "lack," that we can't know who we are unless we buy information about ourselves. When you really think about it, "Who do you think you are?" is a pernicious question that suggests that we don't or can't know who we or to whom we are related without advanced data searches and testing. This existential question used to be a philosophical question; now the answers are provided by databases that acquire more valuable information than they provide in the exchange.
"It's a brilliant business model that exploits consumer naiveté."
As you've said before, consumers are actually paying to be the product because the companies are likely to profit more from selling their genetic data. Could you elaborate?
The largest databases, AncestryDNA and 23andMe, have signed lucrative agreements with biotech companies that pay them for the de-identified data of their customers. What's so valuable is the DNA combined with the family relationships. Consumers provide the family relationships and the companies link and extrapolate the results to larger and larger family trees. Combined with the genetic markers for certain diseases, or increased susceptibility to certain diseases, these databases are very valuable for biotech research.
None of that value will ever be returned to consumers except in the form of for-profit drugs. Ancestry, in particular, has removed all information about its "research partners" from its website, making it very difficult to see how it is profiting from its third-party sales. 23andMe is more open about its "two-sided business model," but encourages consumers to donate their information to science. It's a brilliant business model that exploits consumer naiveté.
A WIRED journalist wrote that "23andMe has been sharing insights gleaned from consented customer data with GSK and at least six other pharmaceutical and biotechnology firms for the past three and a half years." Is this a consumer privacy risk?
I don't see that 23andMe did anything to which consumers didn't consent, albeit through arguably unreadable terms and conditions. The part that worries me more is the 300 phenotype data points that the company has collected on its consumers through longitudinal surveys designed, as Anne Wojcicki, CEO and Co-founder of 23andMe, put it, "to circumvent medical records and just self-report."
Everyone is focused on the DNA, but it's the combination of genetic samples, genealogical information and health records that is the most potent dataset, and 23andMe has figured out a way to extract all three from consumers.
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
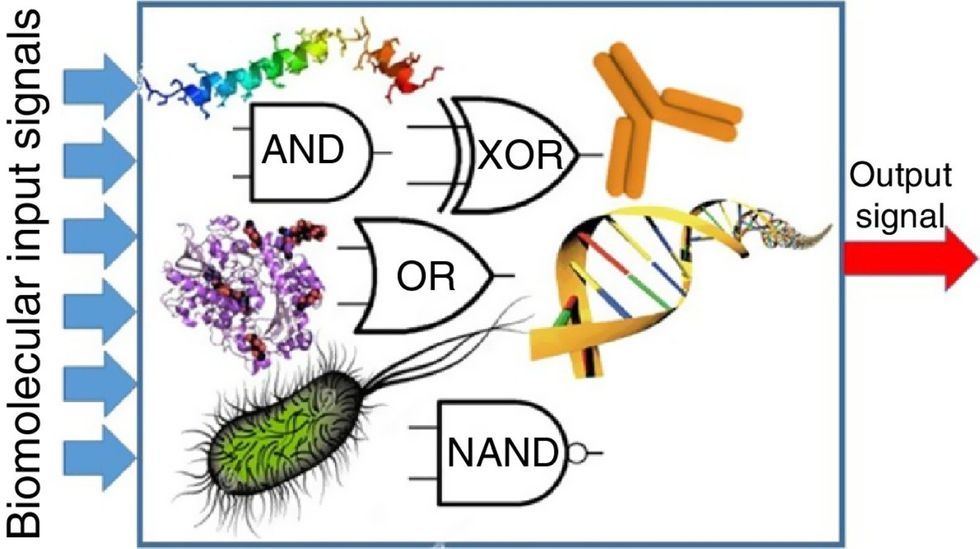
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.