3 Futuristic Biotech Programs the U.S. Government Is Funding Right Now
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Biomedical engineer Kevin Zhao has a sensor in his arm and his chest that monitors his oxygen level in those tissues in real time.
Last month, at a conference celebrating DARPA, the research arm of the Defense Department, FBI Special Agent Edward You declared, "The 21st century will be the revolution of the life sciences."
Biomedical engineer Kevin Zhao has a sensor in his arm and chest that monitors his oxygen level in real time.
Indeed, four years ago, the agency dedicated a new office solely to advancing biotechnology. Its primary goal is to combat bioterrorism, protect U.S. forces, and promote warfighter readiness. But its research could also carry over to improve health care for the general public.
With an annual budget of about $3 billion, DARPA's employees oversee about 250 research and development programs, working with contractors from corporations, universities, and government labs to bring new technologies to life.
Check out these three current programs:
1) IMPLANTABLE SENSORS TO MEASURE OXYGEN, LACTATE, AND GLUCOSE LEVELS IN REAL TIME
Biomedical engineer Kevin Zhao has a sensor in his arm and his chest that monitors his oxygen level in those tissues in real time. With funding from DARPA for the program "In Vivo Nanoplatforms," he developed soft, flexible hydrogels that are injected just beneath the skin to perform the monitoring and that sync to a smartphone app to give the user immediate health insights.
A first-in-man trial for the glucose sensor is now underway in Europe for monitoring diabetics, according to Zhao. Volunteers eat sugary food to spike their glucose levels and prompt the monitor to register the changes.
"If this pans out, with approval from FDA, then consumers could get the sensors implanted in their core to measure their levels of glucose, oxygen, and lactate," Zhao said.
Lactate, especially, interests DARPA because it's a first responder molecule to the onset of trauma, sepsis, and potentially infection.
"The sensor could potentially detect rise of these [body chemistry numbers] and alert the user to prevent onset of dangerous illness."
2) NEAR INSTANTANEOUS VACCINE PROTECTION DURING A PANDEMIC
Traditional vaccines can take months or years to develop, then weeks to become effective once you get it. But when an unknown virus emerges, there's no time to waste.
This program, called P3, envisions a much more ambitious approach to stop a pandemic in its tracks.
"We want to confer near instantaneous protection by doing it a different way – enlist the body as a bioreactor to produce therapeutics," said Col. Matthew Hepburn, the program manager.
So how would it work?
To fight a pandemic, we will need 20,000 doses of a vaccine in 60 days.
If you have antibodies against a certain infection, you'll be protected against that infection. This idea is to discover the genetic code for the antibody to a specific pathogen, manufacture those pieces of DNA and RNA, and then inject the code into a person's arm so the muscle cells will begin producing the required antibodies.
"The amazing thing is that it actually works, at least in animal models," said Hepburn. "The mouse muscles made enough protective antibodies so that the mice were protected."
The next step is to test the approach in humans, which the program will do over the next two years.
But the hard part is actually not discovering the genetic code for highly potent antibodies, according to Hepburn. In fact, researchers already have been able to do so in two to four weeks' time.
"The hard part is once I have an antibody, a large pharma company will say in 2 years, I can make 100-200 doses. Give us 4 years to get to 20,000 doses. That's not good enough," Hepburn said.
To fight a pandemic, we will need 20,000 doses of a vaccine in 60 days.
"We have to fundamentally change the idea that it takes a billion dollars and ten years to make a drug," he concluded. "We're going to do something radically different."
3) RAPID DIAGNOSING OF PATHOGEN EXPOSURE THROUGH EPIGENETICS
Imagine that you come down with a mysterious illness. It could be caused by a virus, bacteria, or in the most extreme catastrophe, a biological agent from a weapon of mass destruction.
What if a portable device existed that could identify--within 30 minutes—which pathogen you have been exposed to and when? It would be pretty remarkable for soldiers in the field, but also for civilians seeking medical treatment.
This is the lofty ambition of a DARPA program called Epigenetic Characterization and Observation, or ECHO.
Its success depends on a biological phenomenon known as the epigenome. While your DNA is relatively immutable, your environment can modify how your DNA is expressed, leaving marks of exposure that register within seconds to minutes; these marks can persist for decades. It's thanks to the epigenome that identical twins – who share identical DNA – can differ in health, temperament, and appearance.

These three mice are genetically identical. Epigenetic differences, however, result in vastly different observed characteristics.
Reading your epigenetic marks could theoretically reveal a time-stamped history of your body's environmental exposures.
Researchers in the ECHO program plan to create a database of signatures for exposure events, so that their envisioned device will be able to quickly scan someone's epigenome and refer to the database to sort out a diagnosis.
"One difficult part is to put a timestamp on this result, in addition to the sign of which exposure it was -- to tell us when this exposure happened," says Thomas Thomou, a contract scientist who is providing technical assistance to the ECHO program manager.
Other questions that remain up in the air for now: Do all humans have the same epigenetic response to the same exposure events? Is it possible to distinguish viral from bacterial exposures? Does dose and duration of exposure affect the signature of epigenome modification?
The program will kick off in January 2019 and is planned to last four years, as long as certain milestones of development are reached along the way. The desired prototype would be a simple device that any untrained person could operate by taking a swab or a fingerprick.
"In an outbreak," says Dr. Thomou, "it will help everyone on the ground immediately to have a rapidly deployable machine that will give you very quick answers to issues that could have far-reaching ramifications for public health safety."
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
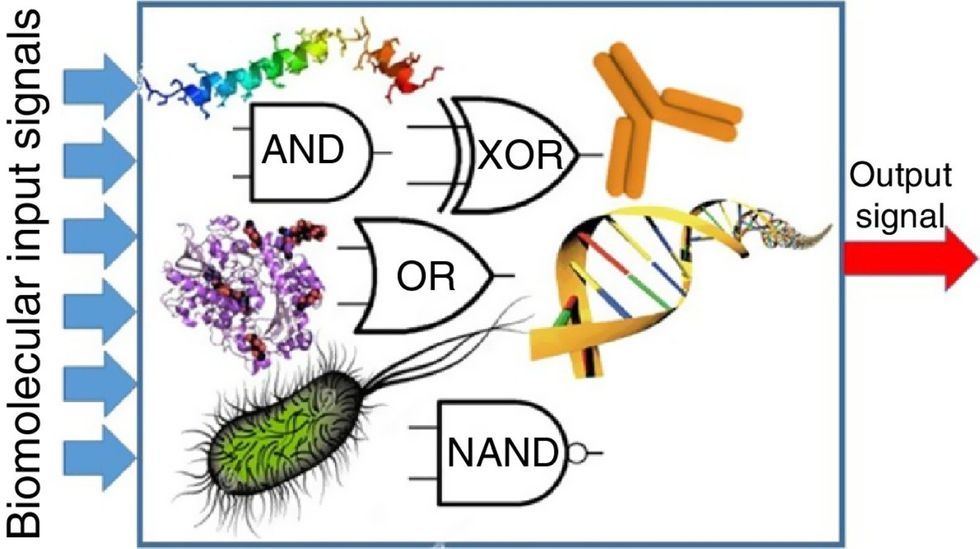
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.