A Single Blood Test May Soon Replace Your Annual Physical
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
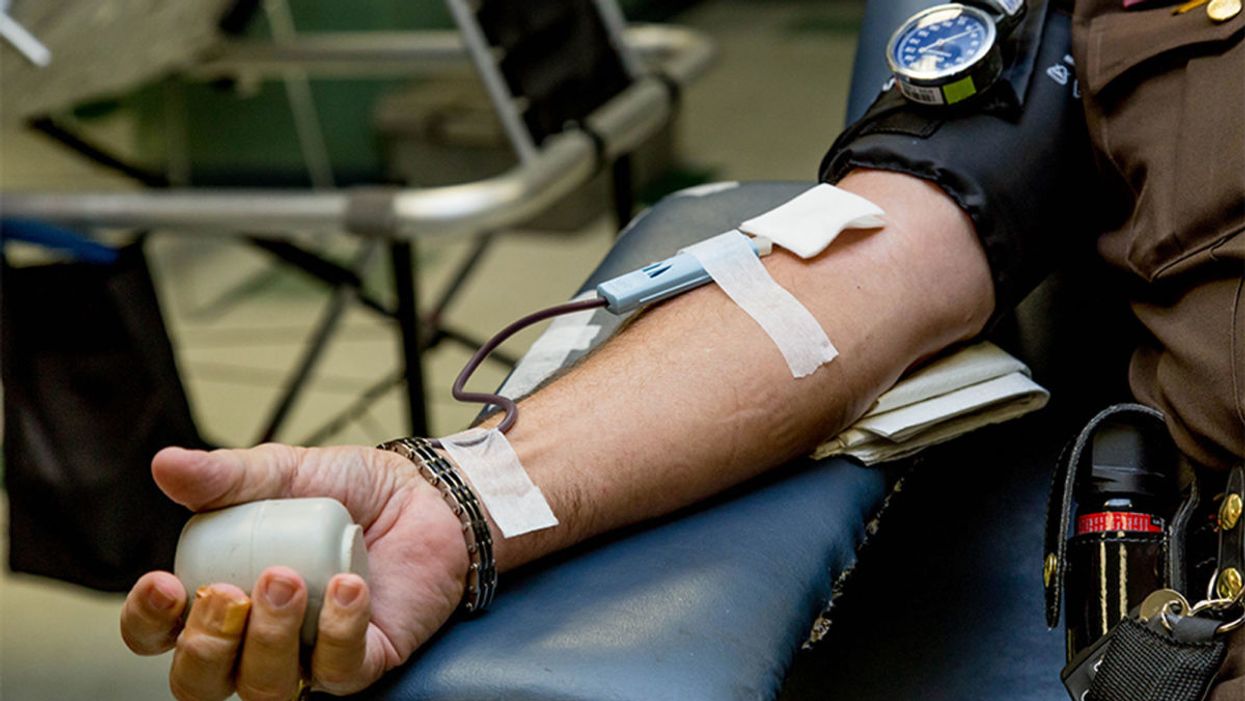
The measurement of proteins in one blood test could provide a comprehensive snapshot of a person's health in the near future.
For all the excitement over "personalized medicine" in the last two decades, its promise has not fully come to pass. Consider your standard annual physical.
Scientists have measured thousands of proteins from a single blood test to assess many individualized health conditions at once.
Your doctor still does a blood test to check your cholesterol and gauge your risk for heart disease by considering traditional risk factors (like smoking, diabetes, blood pressure) — an evaluation that has not changed in decades.
But a high-risk number alone is not enough to tell accurately whether you will suffer from heart disease. It just reflects your risk compared to population-level averages. In other words, not every person with elevated "bad" cholesterol will have a heart attack, so how can doctors determine who truly needs to give up the cheeseburgers and who doesn't?
Now, an emerging area of research may unlock some real-time answers. For the first time, as reported in the journal Nature Medicine last week, scientists have measured thousands of proteins from a single blood test to assess many individualized health conditions at once, including liver and kidney function, diabetes risk, body fat, cardiopulmonary fitness, and even smoking and alcohol consumption. Proteins can give a clear snapshot of how your body is faring at any given moment, as well as a sneak preview at what diseases may be lurking under the surface.
"Years from now," says study co-author Peter Ganz of UCSF, "we will probably be looking back on this paper as a milestone in personalized medicine."
We spoke to Ganz about the significance of this milestone. Our interview has been edited and condensed.
Is this the first study of its kind?
Yes, it is. This is a study where we measured 5,000 proteins at once to look for patterns that could either predict the risk of future diseases or inform the current state of health. Previous to this, people have measured typically one protein at a time, and some of these individual proteins have made it into clinical practice.
An example would be a protein called C-reactive protein, which is a measure of inflammation and is used sometimes in cardiology to predict the risk of future heart attacks. But what's really new is this scale. We wanted to get away from just focusing on one problem that the patient may have at a time, whether it's heart disease or kidney disease, and by measuring a much greater number of proteins, the hope is that we could inform the health of ultimately just about every organ in the body or every tissue. It's a step forward for what I would call "a one-stop shop."
"I'm very excited about personalized medicine through proteins as opposed to genes because you get both the nature and nurture."
Three things get me excited about this. One is the convenience for the patient of a single test to determine many different diseases. The second thing is the healthcare cost savings. We estimated what the cost would be to get these 11 healthcare measures that we reported on using traditional testing and the cost was upwards of 3,000 British pounds. And even though I don't know for sure what the cost of the protein tests would ultimately be, [it could come down to about $50 to $100].
The last thing is that the measurement of proteins is part of what people have called personalized medicine or precision medicine. If you look at risk factors across the population, it may not apply to individuals. In contrast, proteins are downstream of risk factors. So proteins actually tell us whether the traditional risk factors have set in motion the necessary machinery to cause disease. Proteins are the worker bees that regulate what the human body does, and so if you can find some anomalies in the proteins, that may inform us if a disease is likely to be ongoing even in its earliest stages.
Does protein testing have advantages over genetic testing for predicting future health risks?
The problem with genomics is that genes usually don't take care of the environment. It's a blueprint, but your blueprint has no idea what you will be exposed to during your lifetime in terms of the environment and lifestyle that you may choose and medications that you may be on. These are things that proteins can account for. I'm very excited about personalized medicine through proteins as opposed to genes because you get both the nature and nurture as opposed to genomics, which only gives you nature but doesn't account for anything else.
Proteins can also be tracked over time and that's not something you can do with genes. So if your behavior improves, your genes won't change, but your proteins will.
Could this new test become a regular feature of your annual physical?
That's the idea. This would be basically almost a standalone test that you could have done every year. And hopefully you wouldn't need other tests to complement this. This could be your yearly physical.
How much more does it need to be validated before it can enter the clinic and patients can trust the results?
This was a proof-of concept study. To really make this useful, we need to expand from 11 measures of health to a hundred or more health insights, to cover the whole body. And we need to expand this to all racial groups. Three of the five centers in the study were European – all Caucasian – so it's one of our high priorities to find groups of patients with better representation of minorities.
When do you expect doctors to be routinely giving this test to patients?
Much closer to five years than 20 years. We're scaling up from 11 disease states to 100, and many of those studies are underway. Results should be done within three to five years.
Do you think insurance will cover it?
Good question. I have been approached by an insurance company that wanted to understand the product better – a major insurer, with the possibility that this could actually be cost saving.
I have to ask you a curveball -- do you think that the downfall of Theranos will make consumers hesitant to trust a new technology that relies on using a single blood sample to screen for multiple health risks?
[Laughs] You're not the first person to ask me that today. I actually got a call from Elizabeth Holmes [in 2008 when I was at Harvard]. I met with her for an afternoon and met her team two more times. I gave them advice that they completely disregarded.
In many ways, what we do is diametrically opposite to Theranos. They had a culture of secrecy, and what we do is about openness. We publish, like this paper in Nature Medicine, to show the scientific details. Our supplement is much longer than the typical academic paper. We reveal everything we know. A lot of the research we do is funded by [the National Institutes of Health], and they have strict expectations about data sharing. So we agree to make the data available on a public website. If there is something we haven't done with the data, others can do it.
So you're saying that this is not another Theranos.
No, God forbid. We hope to be the opposite.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A company uses AI to fight muscle loss and unhealthy aging
In December 2022, a company called BioAge Labs published findings on a drug that worked to prevent muscular atrophy, or the loss of muscle strength and mass, in older people.
There’s a growing need to slow down the aging process. The world’s population is getting older and, according to one estimate, 80 million Americans will be 65 or older by 2040. As we age, the risk of many chronic diseases goes up, from cancer to heart disease to Alzheimer’s.
BioAge Labs, a company based in California, is using genetic data to help people stay healthy for longer. CEO Kristen Fortney was inspired by the genetics of people who live long lives and resist many age-related diseases. In 2015, she started BioAge to study them and develop drug therapies based on the company’s learnings.
The team works with special biobanks that have been collecting blood samples and health data from individuals for up to 45 years. Using artificial intelligence, BioAge is able to find the distinctive molecular features that distinguish those who have healthy longevity from those who don’t.
In December 2022, BioAge published findings on a drug that worked to prevent muscular atrophy, or the loss of muscle strength and mass, in older people. Much of the research on aging has been in worms and mice, but BioAge is focused on human data, Fortney says. “This boosts our chances of developing drugs that will be safe and effective in human patients.”
How it works
With assistance from AI, BioAge measures more than 100,000 molecules in each blood sample, looking at proteins, RNA and metabolites, or small molecules that are produced through chemical processes. The company uses many techniques to identify these molecules, some of which convert the molecules into charged atoms and then separating them according to their weight and charge. The resulting data is very complex, with many thousands of data points from patients being followed over the decades.
BioAge validates its targets by examining whether a pathway going awry is actually linked to the development of diseases, based on the company’s analysis of biobank health records and blood samples. The team uses AI and machine learning to identify these pathways, and the key proteins in the unhealthy pathways become their main drug targets. “The approach taken by BioAge is an excellent example of how we can harness the power of big data and advances in AI technology to identify new drugs and therapeutic targets,” says Lorna Harries, a professor of molecular genetics at the University of Exeter Medical School.
Martin Borch Jensen is the founder of Gordian Biotechnology, a company focused on using gene therapy to treat aging. He says BioAge’s use of AI allows them to speed up the process of finding promising drug candidates. However, it remains a challenge to separate pathologies from aspects of the natural aging process that aren’t necessarily bad. “Some of the changes are likely protective responses to things going wrong,” Jensen says. “Their data doesn’t…distinguish that so they’ll need to validate and be clever.”
Developing a drug for muscle loss
BioAge decided to focus on muscular atrophy because it affects many elderly people, making it difficult to perform everyday activities and increasing the risk of falls. Using the biobank samples, the team modeled different pathways that looked like they could improve muscle health. They found that people who had faster walking speeds, better grip strength and lived longer had higher levels of a protein called apelin.
Apelin is a peptide, or a small protein, that circulates in the blood. It is involved in the process by which exercise increases and preserves muscle mass. BioAge wondered if they could prevent muscular atrophy by increasing the amount of signaling in the apelin pathway. Instead of the long process of designing a drug, they decided to repurpose an existing drug made by another biotech company. This company, called Amgen, had explored the drug as a way to treat heart failure. It didn’t end up working for that purpose, but BioAge took note that the drug did seem to activate the apelin pathway.
BioAge tested its new, repurposed drug, BGE-105, and, in a phase 1 clinical trial, it protected subjects from getting muscular atrophy compared to a placebo group that didn’t receive the drug. Healthy volunteers over age 65 received infusions of the drug during 10 days spent in bed, as if they were on bed rest while recovering from an illness or injury; the elderly are especially vulnerable to muscle loss in this situation. The 11 people taking BGE-105 showed a 100 percent improvement in thigh circumference compared to 10 people taking the placebo. Ultrasound observations also revealed that the group taking the durg had enhanced muscle quality and a 73 percent increase in muscle thickness. One volunteer taking BGE-105 did have muscle loss compared to the the placebo group.
Heather Whitson, the director of the Duke University Centre for the study of aging and human development, says that, overall, the results are encouraging. “The clinical findings so far support the premise that AI can help us sort through enormous amounts of data and identify the most promising points for beneficial interventions.”
More studies are needed to find out which patients benefit the most and whether there are side effects. “I think further studies will answer more questions,” Whitson says, noting that BGE-105 was designed to enhance only one aspect of physiology associated with exercise, muscle strength. But exercise itself has many other benefits on mood, sleep, bones and glucose metabolism. “We don’t know whether BGE-105 will impact these other outcomes,” she says.
The future
BioAge is planning phase 2 trials for muscular atrophy in patients with obesity and those who have been hospitalized in an intensive care unit. Using the data from biobanks, they’ve also developed another drug, BGE-100, to treat chronic inflammation in the brain, a condition that can worsen with age and contributes to neurodegenerative diseases. The team is currently testing the drug in animals to assess its effects and find the right dose.
BioAge envisions that its drugs will have broader implications for health than treating any one specific disease. “Ultimately, we hope to pioneer a paradigm shift in healthcare, from treatment to prevention, by targeting the root causes of aging itself,” Fortney says. “We foresee a future where healthy longevity is within reach for all.”
How old fishing nets turn into chairs, car mats and Prada bags
A company called Aquafil is turning the fibers from fishing nets and old carpets into new threads for chairs, car mats, bikinis and Prada bags.
Discarded nylon fishing nets in the oceans are among the most harmful forms of plastic pollution. Every year, about 640,000 tons of fishing gear are left in our oceans and other water bodies to turn into death traps for marine life. London-based non-profit World Animal Protection estimates that entanglement in this “ghost gear” kills at least 136,000 seals, sea lions and large whales every year. Experts are challenged to estimate how many birds, turtles, fish and other species meet the same fate because the numbers are so high.
Since 2009, Giulio Bonazzi, the son of a small textile producer in northern Italy, has been working on a solution: an efficient recycling process for nylon. As CEO and chairman of a company called Aquafil, Bonazzi is turning the fibers from fishing nets – and old carpets – into new threads for car mats, Adidas bikinis, environmentally friendly carpets and Prada bags.
For Bonazzi, shifting to recycled nylon was a question of survival for the family business. His parents founded a textile company in 1959 in a garage in Verona, Italy. Fifteen years later, they started Aquafil to produce nylon for making raincoats, an enterprise that led to factories on three continents. But before the turn of the century, cheap products from Asia flooded the market and destroyed Europe’s textile production. When Bonazzi had finished his business studies and prepared to take over the family company, he wondered how he could produce nylon, which is usually produced from petrochemicals, in a way that was both successful and ecologically sustainable.
The question led him on an intellectual journey as he read influential books by activists such as world-renowned marine biologist Sylvia Earle and got to know Michael Braungart, who helped develop the Cradle-to-Cradle ethos of a circular economy. But the challenges of applying these ideologies to his family business were steep. Although fishing nets have become a mainstay of environmental fashion ads—and giants like Dupont and BASF have made breakthroughs in recycling nylon—no one had been able to scale up these efforts.
For ten years, Bonazzi tinkered with ideas for a proprietary recycling process. “It’s incredibly difficult because these products are not made to be recycled,” Bonazzi says. One complication is the variety of materials used in older carpets. “They are made to be beautiful, to last, to be useful. We vastly underestimated the difficulty when we started.”
Soon it became clear to Bonazzi that he needed to change the entire production process. He found a way to disintegrate old fibers with heat and pull new strings from the discarded fishing nets and carpets. In 2022, his company Aquafil produced more than 45,000 tons of Econyl, which is 100% recycled nylon, from discarded waste.
More than half of Aquafil’s recyclate is from used goods. According to the company, the recycling saves 90 percent of the CO2 emissions compared to the production of conventional nylon. That amounts to saving 57,100 tons of CO2 equivalents for every 10,000 tons of Econyl produced.
Bonazzi collects fishing nets from all over the world, including Norway and Chile—which have the world’s largest salmon productions—in addition to the Mediterranean, Turkey, India, Japan, Thailand, the Philippines, Pakistan, and New Zealand. He counts the government leadership of Seychelles as his most recent client; the island has prohibited ships from throwing away their fishing nets, creating the demand for a reliable recycler. With nearly 3,000 employees, Aquafil operates almost 40 collection and production sites in a dozen countries, including four collection sites for old carpets in the U.S., located in California and Arizona.
First, the dirty nets are gathered, washed and dried. Bonazzi explains that nets often have been treated with antifouling agents such as copper oxide. “We recycle the coating separately,” he says via Zoom from his home near Verona. “Copper oxide is a useful substance, why throw it away?”
Still, only a small percentage of Aquafil’s products are made from nets fished out of the ocean, so your new bikini may not have saved a strangled baby dolphin. “Generally, nylon recycling is a good idea,” says Christian Schiller, the CEO of Cirplus, the largest global marketplace for recyclates and plastic waste. “But contrary to what consumers think, people rarely go out to the ocean to collect ghost nets. Most are old, discarded nets collected on land. There’s nothing wrong with this, but I find it a tad misleading to label the final products as made from ‘ocean plastic,’ prompting consumers to think they’re helping to clean the oceans by buying these products.”
Aquafil gets most of its nets from aqua farms. Surprisingly, one of Aquafil’s biggest problems is finding enough waste. “I know, it’s hard to believe because waste is everywhere,” Bonazzi says. “But we need to find it in reliable quantity and quality.” He has invested millions in establishing reliable logistics to source the fishing nets. Then the nets get shredded into granules that can be turned into car mats for the new Hyundai Ioniq 5 or a Gucci swimsuit.
The process works similarly with carpets. In the U.S. alone, 3.5 billion pounds of carpet are discarded in landfills every year, and less than 3 percent are currently recycled. Aquafil has built a recycling plant in Phoenix to help divert 12,500 tons of carpets from the landfill every year. The carpets are shredded and deconstructed into three components: fillers such as calcium carbonate will be reused in the cement industry, synthetic fibers like polypropylene can be used for engineering plastics, and nylon. Only the pelletized nylon gets shipped back to Europe for the production of Econyl. “We ship only what’s necessary,” Bonazzi says. Nearly 50 percent of his nylon in Italy and Slovenia is produced from recyclate, and he hopes to increase the percentage to two-thirds in the next two years.
His clients include Interface, the leading world pioneer for sustainable flooring, and many other carpet producers plus more than 2500 fashion labels, including Gucci, Prada, Patagonia, Louis Vuitton, Adidas and Stella McCartney. “Stella McCartney just introduced a parka that’s made 100 percent from Econyl,” Bonazzi says. “We’re also in a lot of sportswear because Nylon is a good fabric for swimwear and for yoga clothes.” Next, he’s looking into sunglasses and chairs made with Econyl - for instance, the flexible ergonomic noho chair, designed by New Zealand company Formway.
“When I look at a landfill, I see a gold mine," Bonazzi says.
“Bonazzi decided many years ago to invest in the production of recycled nylon though industry giants halted similar plans after losing large investments,” says Anika Herrmann, vice president of the German Greentech-competitor Camm Solutions, which creates bio-based polymers from cane sugar and other ag waste. “We need role models like Bonazzi who create sustainable solutions with courage and a pioneering spirit. Like Aquafil, we count on strategic partnerships to enable fast upscaling along the entire production chain.”
Bonazzi’s recycled nylon is still five to 10 percent more expensive than conventionally produced material. However, brands are increasingly bending to the pressure of eco-conscious consumers who demand sustainable fashion. What helped Bonazzi was the recent rise of oil prices and the pressure on industries to reduce their carbon footprint. Now Bonazzi says, “When I look at a landfill, I see a gold mine.”
Ideally, the manufacturers take the products back when the client is done with it, and because the nylon can theoretically be reused nearly infinitely, the chair or bikini could be made into another chair or bikini. “But honestly,” Bonazzi half-jokes, “if someone returns a McCartney parka to me, I’ll just resell it because it’s so expensive.”
The next step: Bonazzi wants to reshape the entire nylon industry by pivoting from post-consumer nylon to plant-based nylon. In 2017, he began producing “nylon-6,” together with Genomatica in San Diego. The process uses sugar instead of petroleum. “The idea is to make the very same molecule from sugar, not from oil,” he says. The demonstration plant in Ljubljana, Slovenia, has already produced several hundred tons of nylon, and Genomatica is collaborating with Lululemon to produce plant-based yoga wear.
Bonazzi acknowledges that his company needs a few more years before the technology is ready to meet his ultimate goal, producing only recyclable products with no petrochemicals, low emissions and zero waste on an industrial scale. “Recycling is not enough,” he says. “You also need to produce the primary material in a sustainable way, with a low carbon footprint.”

