How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next

Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
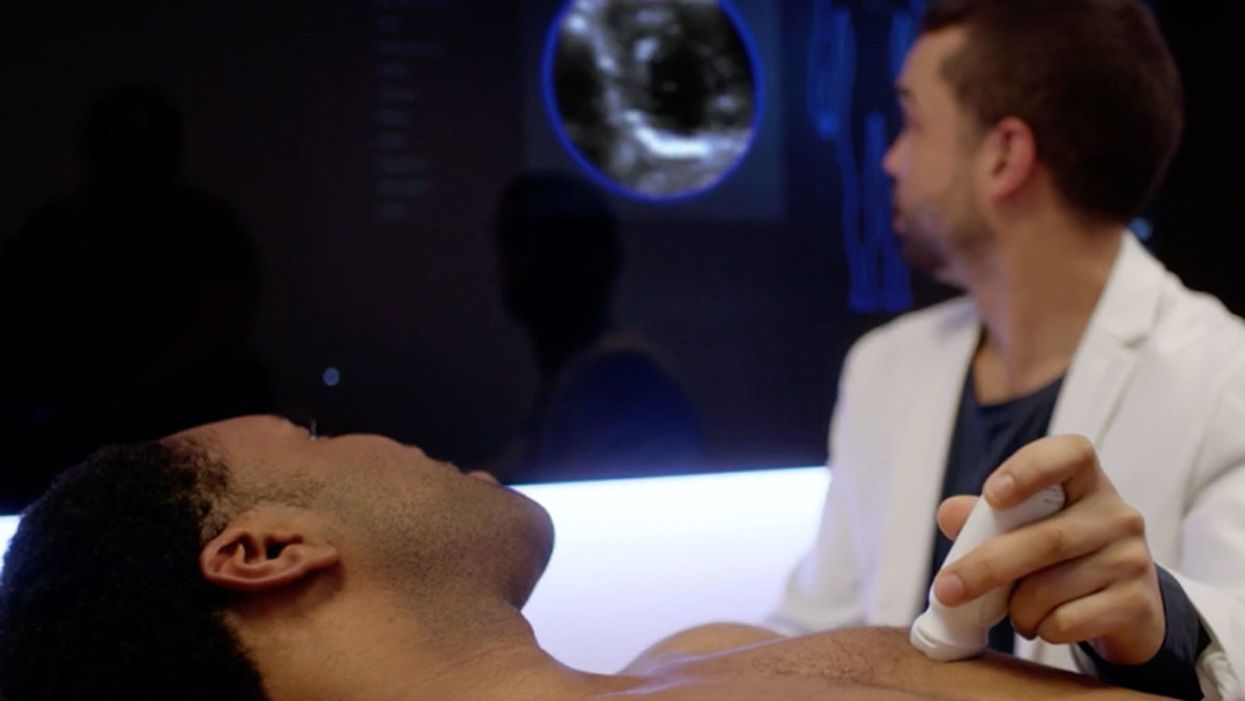
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”
At the “Apple Store of Doctor’s Offices,” Preventive Care Is High Tech. Is it Worth $150 a Month?
A patient getting examined at health startup Forward.
What if going to the doctor's office could be … nice?
If you didn't have to wait for your appointment, but were ushered right in; if your medical data was all collated and easily searchable on an iPhone app; if a remote scribe took notes while you spoke with your doctor so you could make eye contact with them; if your doctor didn't seem horribly rushed.
Would you go to the doctor to get help staying healthy, rather than just to stop being sick?
Would that change the way you thought about your health? Would you go to the doctor to get help staying healthy, rather than just to stop being sick? And would that, in the long run, be much better for you?
Those are the animating questions for Forward, a healthcare startup devoted to preventive care. Led by founder Adrian Aoun, formerly of Google/Sidewalk labs, Forward opened its first office in San Francisco in 2016 and has since expanded to Los Angeles, Orange County, New York, and Washington, D.C., with a San Diego location opening soon.
It's been described as the "Apple Store of doctor's offices," which in some ways is a reaction to Forward's vibe: Patients have described the offices as having blonde wood, minimalist design, sparkling water on tap — and lots of high-tech gadgets, like the full-body scanner that replaces the standard scale and stethoscope.

The interior of a Forward office.
(Courtesy Forward)
The more crucial difference, though, is its model of care. Forward doesn't take insurance. Instead, patients, or "members," pay a flat $149 per month, along the lines of a subscription service like Netflix or a gym membership. That fee covers visits, messaging with medical staff through the Forward app, the use of a wearable (like a Fitbit or a sleep tracker) if the physician recommends it, plus any bloodwork or diagnostic tests run in the on-site labs. (The company declined to disclose how many people have signed up for memberships.)
Predictability is Forward's other significant, distinguishing feature: No surprise co-pays, or extra charges showing up on a billing statement months later. Everything is wrapped up in the $149 membership fee, unless the physician recommends visiting an outside specialist.
That caveat isn't a small one. It's important to note that Forward is in no way meant to replace standard health insurance. The service is strictly focused on preventive care, so it wouldn't be much use in case of an emergency; it's meant to help people, as far as is possible, avoid that emergency at all.
Ani Okkasian's family recently went through such an emergency. Her 62-year-old father, an active and seemingly healthy man living with diabetes, had been feeling unwell for a while, but struggled to receive constructive follow-up or tests from his doctor. It finally emerged that his liver was severely damaged, and he suffered a stroke — the risk of which can be elevated by liver disease. He seemed to deteriorate completely within mere weeks, Okkasian said, and in January he passed away.
"He was someone who'd go to the doctor regularly and listen to what they said and follow it," Okkasian said. "I shouldn't have had to bury my father at 62. I still believe to my core that his death could have been avoided if the primary care was adequate."
"I could tell that the people who designed [Forward] had lost someone to the legacy system; it was so streamlined and so much clearer."
Okkasian began researching, looking for a better alternative, and discovered Forward. Founder Aoun lost his grandfather to a heart attack; his brother's heart attack at age 31 was the impetus to start Forward.
"I could tell that that was the genesis," Okkasian said. "Having just lost someone, and having had to deal with different aspects of the healthcare industry — how complicated and convoluted that all is — I could tell that the people who designed [Forward] had lost someone to the legacy system; it was so streamlined and so much clearer."
So Who Is Forward For?
The Affordable Care Act mandates that evidence-based preventive care must be covered by insurers without any cost to the patient. Today, 30 million Americans are still living without health insurance; but for most of the population, cost shouldn't prevent access to standard, preventive care, says Benjamin Sommers, a physician and professor at the Harvard T.H. Chan School of Public Health who has studied the effect of the ACA on preventive care access.
For Okkasian and her family, it wasn't a lack of access to primary care that was at issue; it was the quality of that primary care. In 2019, that's probably true for a lot of people.
"How come all other industries have been disturbed except the medical industry?" Okkasian asked. "It's disturbing the most people. We're so advanced in so many ways, but when it comes to the healthcare system, we're not prioritizing the wellness of a person."
Is Forward the answer? Well, probably not for everyone. Its office are only in a handful of cities, and there are limits to how scalable it would be; it's unavoidable that the $149 per month charge restricts access for a lot of people. Those who have insurance through their employer might have a flexible spending account (FSA) that would cover some or all of the membership fee, and Forward has said that 15 percent of their early members came from underserved communities and were offered free plans; but for many others, that's just an unworkable extra cost.
Sommers also sounded a dubious note about a maximalist attitude toward data collection.
"Even though some patients may think that 'more is always better' — more testing, more screening, etc. — this isn't true," he said. "Some types of cancer screening, ovarian cancer screening for instance, are actually harmful or of no benefit, because studies have shown that they don't improve survival or health outcomes, but can lead to unnecessary testing, pain, false positives, anxiety, and other side effects.
"It's really great for people who are in good health, looking to make it better."
"I'm generally skeptical of efforts to charge people more to get 'extra testing' that isn't currently supported by the medical evidence," he added.
But relatively healthy people who want to take a more active approach to their health — or people who have frequent testing needs, like those using the HIV-prevention drug PrEP, and want to avoid co-pays — might benefit from the on-demand, low-friction experience that Forward offers.
"It's really great for people who are in good health, looking to make it better," Okkasian said. "Your experience is simplified to a point where you feel empowered, not scared."
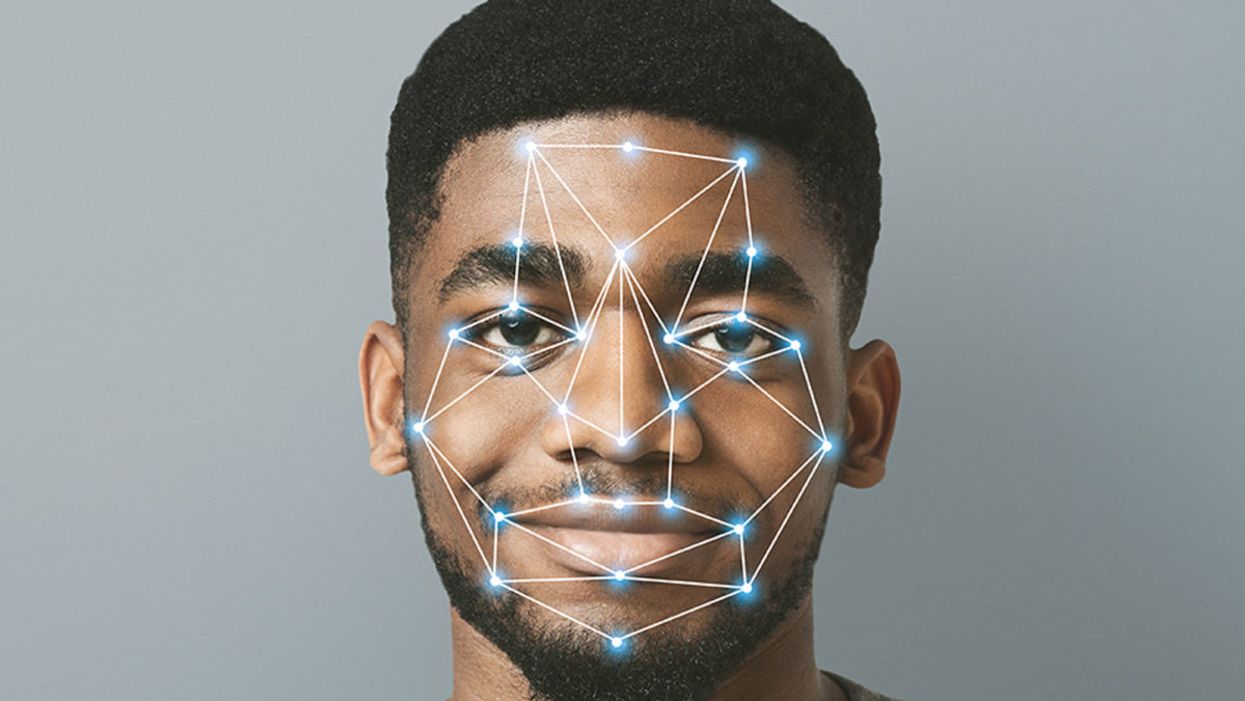
Facial Recognition Can Reduce Racial Profiling and False Arrests
The use of face recognition technology is expanding exponentially right now.
[Editor's Note: This essay is in response to our current Big Question, which we posed to experts with different perspectives: "Do you think the use of facial recognition technology by the police or government should be banned? If so, why? If not, what limits, if any, should be placed on its use?"]
Opposing facial recognition technology has become an article of faith for civil libertarians. Many who supported the bans in cities like San Francisco and Oakland have declared the technology to be inherently racist and abusive.
The greatest danger would be to categorically oppose this technology and pretend that it will simply go away.
I have spent my career as a criminal defense attorney and a civil libertarian -- and I do not fear it. Indeed, I see it as positive so long as it is appropriately regulated and controlled.
We are living in the beginning of a biometric age, where technology uses our physical or biological characteristics for a variety of products and services. It holds great promises as well as great risks. The greatest danger, however, would be to categorically oppose this technology and pretend that it will simply go away.
This is an age driven as much by consumer as it is government demand. Living in denial may be emotionally appealing, but it will only hasten the creation of post-privacy world. If we do not address this emerging technology, movements in public will increasingly result in instant recognition and even tracking. It is the type of fish-bowl society that strips away any expectation of privacy in our interactions and associations.
The biometrics field is expanding exponentially, largely due to the popularity of consumer products using facial recognition technology (FRT) -- from the iPhone program to shopping ones that recognize customers.
But the privacy community is losing this battle because it is using the privacy rationales and doctrines forged in the earlier electronic surveillance periods. Just as generals are often accused of planning to fight the last war, civil libertarians can sometimes cling to past models despite their decreasing relevance in the current world.
I see FRT as having positive implications that are worth pursuing. When properly used, biometrics can actually enhance privacy interests and even reduce racial profiling by reducing false arrests and the warrantless "patdowns" allowed by the Supreme Court. Bans not only deny police a technology widely used by businesses, but return police to the highly flawed default of "eye balling" suspects -- a system with a considerably higher error rate than top FRT programs.
Officers are often wrong and stop a great number of suspects in the hopes of finding a wanted felon.
A study in Australia showed that passport officers who had taken photographs of subjects in ideal conditions nonetheless experienced high error rates when identifying them shortly afterward, including 14 percent false acceptance rates. Currently, officers stop suspects based on their memory from seeing a photograph days or weeks earlier. They are often wrong and stop a great number of suspects in the hopes of finding a wanted felon. The best FRT programs achieve an astonishing accuracy rate, though real-world implementation has challenges that must be addressed.
One legitimate concern raised in early studies showed higher error rates in recognitions for certain groups, particularly African American women. An MIT study finding that error rate prompted major improvements in the algorithms as well as training changes to greatly reduce the frequency of errors. The issue remains a concern, but there is nothing inherently racist in algorithms. These are a set of computer instructions that isolate and process with the parameters and conditions set by creators.
To be sure, there is room for improvement in some algorithms. Tests performed by the American Civil Liberties Union (ACLU) reportedly showed only an 80 percent accuracy rate in comparing mug shots to pictures of members of Congress when using Amazon's "Rekognition" system. It recently showed the same 80 percent rate in doing the same comparison to members of the California legislators.
However, different algorithms are available with differing levels of performance. Moreover, these products can be set with a lower discrimination level. The fact is that the top algorithms tested by the National Institute of Standards and Technology showed that their accuracy rate is greater than 99 percent.
The greatest threat of biometric technologies is to democratic values.
Assuming a top-performing algorithm is used, the result could be highly beneficial for civil liberties as opposed to the alternative of "eye balling" suspects. Consider the Boston Bombing where police declared a "containment zone" and forced families into the street with their hands in the air.
The suspect, Dzhokhar Tsarnaev, moved around Boston and was ultimately found outside the "containment zone" once authorities abandoned near martial law. He was caught on some surveillance systems but not identified. FRT can help law enforcement avoid time-consuming area searches and the questionable practice of forcing people out of their homes to physically examine them.
If we are to avoid a post-privacy world, we will have to redefine what we are trying to protect and reconceive how we hope to protect it. In my view, the greatest threat of biometric technologies is to democratic values. Authoritarian nations like China have made huge investments into FRT precisely because they know that the threat of recognition in public deters citizens from associating or interacting with protesters or dissidents. Recognition changes conduct. That chilling effect is what we have the worry about the most.
Conventional privacy doctrines do not offer much protection. The very concept of "public privacy" is treated as something of an oxymoron by courts. Public acts and associations are treated as lacking any reasonable expectation of privacy. In the same vein, the right to anonymity is not a strong avenue for protection. We are not living in an anonymous world anymore.
Consumers want products like FaceFind, which link their images with others across social media. They like "frictionless" transactions and authentications using faceprints. Despite the hyperbole in places like San Francisco, civil libertarians will not succeed in getting that cat to walk backwards.
The basis for biometric privacy protection should not be focused on anonymity, but rather obscurity. You will be increasingly subject to transparency-forcing technology, but we can legislatively mandate ways of obscuring that information. That is the objective of the Biometric Privacy Act that I have proposed in recent research. However, no such comprehensive legislation has passed through Congress.
The ability to spot fraudulent entries at airports or recognizing a felon in flight has obvious benefits for all citizens.
We also need to recognize that FRT has many beneficial uses. Biometric guns can reduce accidents and criminals' conduct. New authentications using FRT and other biometric programs could reduce identity theft.
And, yes, FRT could help protect against unnecessary police stops or false arrests. Finally, and not insignificantly, this technology could stop serious crimes, from terrorist attacks to the capturing of dangerous felons. The ability to spot fraudulent entries at airports or recognizing a felon in flight has obvious benefits for all citizens.
We can live and thrive in a biometric era. However, we will need to bring together civil libertarians with business and government experts if we are going to control this technology rather than have it control us.
[Editor's Note: Read the opposite perspective here.]