How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next

Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”
Scientists Just Created Liquid Solar Power That Can Be Stored for 18 Years
A team of Swedish scientists has gathered solar power so pure, that until recently, capturing it was an impossibility.
Look no further than this week's climate strikes for evidence that millions of people are passionate about curbing global warming.
Unlike relatively limited solar panel energy storage, norbornadiene can potentially maintain its potency for years.
But even potential solutions, like alternative meats, have their own challenges. Some scientists are putting their focus on the sun to help balance out our energy consumption.
In fact, they are gathering solar power so pure that, until recently, capturing it was an impossibility.
The Lowdown
A group of Swedish scientists has created a liquid called norbornadiene. This liquid sunshine can capture up to 30 percent of raw solar power. To put it in perspective, the best publicly available solar panels can harness 21 percent. Norbornadiene would bring in about 50 percent more power – a significant difference in energy efficiency.
Most notably, unlike relatively limited solar panel energy storage, norbornadiene can potentially maintain its potency for years. We could have the ability to collect and store premium solar power, making it easier for current and future generations to use fossil and nuclear fuel alternatives.
"The norbornadiene molecules that we have made have very good properties, in terms of solar energy capture efficiency, storage time and energy density," says team lead Dr. Kasper Moth-Poulson of the Chamlers University of Technology. "They can store energy without the need for insulation materials for 18 or more years."
Next Up
Swedish scientist Moth-Poulsen and his team have been testing the norbornadiene on the physics building roof at the Chalmers University of Technology. Once activated, it heats up to just below boiling and provides enough power to be useful.
The energy density is 250 watt-hours per kilogram, twice the strength of Tesla's popular Powerall battery.
It requires potentially toxic solvents, like a cobalt-based activator, to transform into its full potential. The team is currently trying to find less-hazardous catalysts to help transform the norbornadiene to its active form, quadricyclane. Exposing it to sunlight is the main way to reactivate the norbornadiene's power. Over time, scientists will likely make it more efficient with less toxic agents.
The energy density is 250 watt-hours per kilogram, twice the strength of Tesla's popular Powerall battery.
Open Questions
The biggest question is safety, perceived or otherwise: Are you ready to drive around with 250 kWh of pure solar in your Hyundai? Norbornadiene may be stable in a hermetically sealed lab, but sculpting it for everyday use requires another level of security.
The half-life of the sunshine power is also an estimate, too. The challenge with new scientific substances is you don't know how the matter will evolve over time. It is easy to be overly optimistic about this one discovery being the key to our energy needs. For the time being, it is wiser to look at norbornadiene as a progressive step rather than a revolutionary one.
Even at its least effective, norbornadiene and its related material is a step toward us utilizing the one natural resource that won't run out for generations. In the short-term, a stable form of it could offset our fossil and nuclear fuel use and even help lower the carbon footprint made by long-distance transportation. It will be fascinating to see what future aircraft builders, home designers and even car manufacturers do as the solar technology conversation heats up.
Moth-Poulsen wants norbornadiene to be a definitive part of the climate change puzzle.
"I hope that in five years, we will see the first products based on our molecules and could help mitigate the daily variations in temperature," he says. "This will lead to increased thermal comfort and reduced energy consumption for heating and cooling."
Virtual Reality is Making Medical Care for Kids Less Scary and Painful
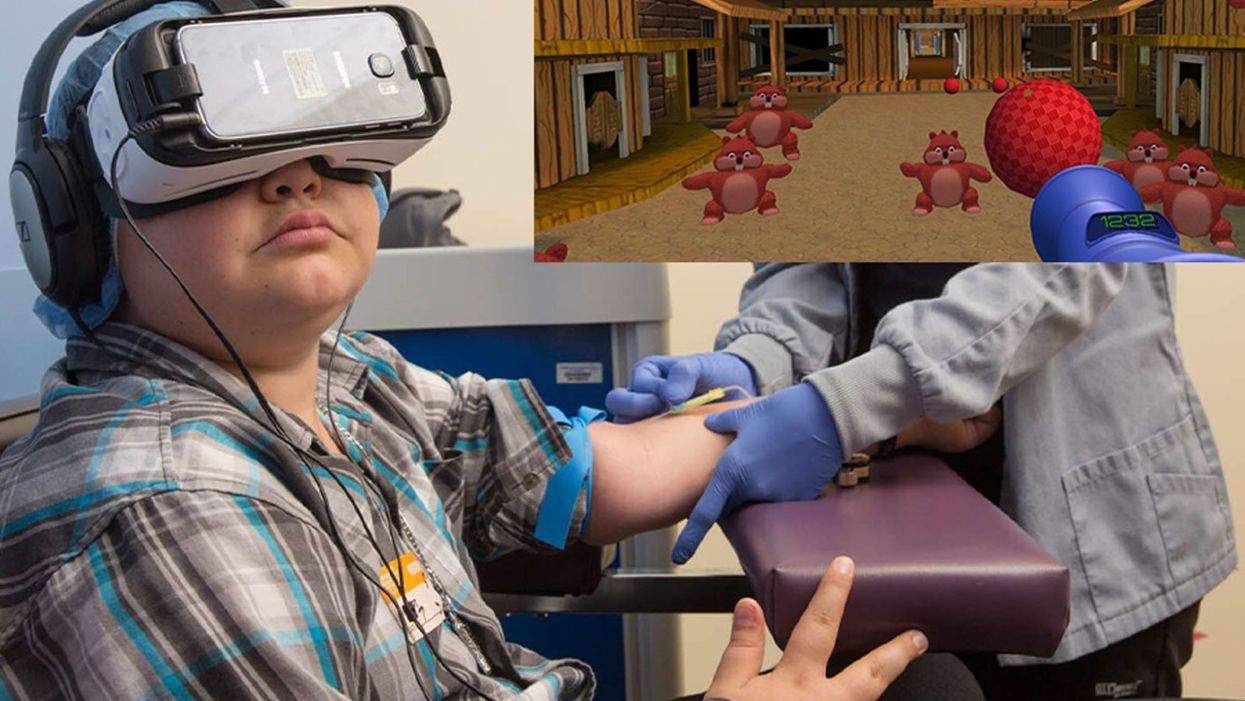
A patient at Children's Hospital Los Angeles (Courtesy of Children's Hospital Los Angeles) plus Bear Blast, developed by AppliedVR.
A blood draw is not normally a fun experience, but these days, virtual reality technology is changing that.
Instead of watching a needle go into his arm, a child wearing a VR headset at Children's Hospital Los Angeles can play a game throwing balls at cartoon bears. In Seattle, at the University of Washington, a burn patient can immerse herself in a soothing snow scene. And at the University of Miami Hospital, a five-minute skin biopsy can become an exciting ride at an amusement park.
VR is transforming once-frightening medical encounters for kids, from blood draws to biopsies to pre-surgical prep, into tolerable ones.
It's literally a game changer, says pediatric neurosurgeon Kurtis Auguste, who uses the tool to help explain pending operations to his young patients and their families. The virtual reality 3-D portrait of their brain is recreated from an MRI, originally to help plan the surgery. The image of normally bland tissue is painted with false colors to better see the boundaries and anomalies of each component. It can be rotated, viewed from every possible angle, zoomed in and out; incisions can be made and likely results anticipated. Auguste has extended its use to patients and families.
"The moment you put these headsets on the kids, we immediately have a link, because honestly, this is how they communicate with each other," says Auguste. "We're all sitting around the table playing games. It's really bridged the distance between me, the pediatric specialist, and my patients" at the Benioff Children's Hospital Oakland, now affiliated with the University of California San Francisco School of Medicine.
The VR experience engages people where they are, immersing them in the environment rather than lecturing them. And it seems to work in all environments, across age and cultural differences, leading to a better grasp of what will be undertaken. That understanding is crucial to meaningful informed consent for surgery. It is particularly relevant for safety-net hospitals, which includes most children's hospitals, because often members of the families were born elsewhere and may have limited understanding of English, not to mention advanced medicine.
Targeting pain
"We're trying to target ways that we can decrease pain, anxiety, fear – what people usually experience as a function of a needle," says Jeffrey Gold, a pioneer in adapting VR at Children's Hospital Los Angeles. He ran the pain clinic there and in 2004 initially focused on phlebotomy, simple blood draws. Many of their kids require frequent blood draws to monitor serious chronic conditions such as diabetes, HIV infection, sickle cell disease, and other conditions that affect the heart, liver, kidneys and other organs.
The scientific explanation of how VR works for pain relief draws upon two basic principles of brain function. The first is "top down inhibition," Gold explains. "We all have the inherent capacity to turn down signals once we determine that signal is no longer harmful, dangerous, hurtful, etc. That's how our brain operates on purpose. It's not just a distraction, it's actually your brain stopping the pain signal at the spinal cord before it can fire all the way up to the frontal lobe."
Second is the analgesic effect from endorphins. "If you're in a gaming environment, and you're having fun and you're laughing and giggling, you are actually releasing endorphins...a neurochemical reaction at the synaptic level of the brain," he says.
Part of what makes VR effective is "what's called a cognitive load, where you have to actually learn something and do something," says Gold. He has worked with developers on a game call Bear Blast, which has proven to be effective in a clinical trial for mitigating pain. But he emphasizes, it is not a one-size-fits all; the programs and patients need to be evaluated to understand what works best for each case.
Gold was a bit surprised to find that VR "actually facilitates quicker blood draws," because the staff doesn't have to manage the kids' anxiety, so "they require fewer needle sticks." The kids, parents, and staff were all having a good time, "and that's a big win when everybody is benefiting." About two years ago the hospital made VR an option that patients can request in the phlebotomy lab, and about half of kids age 4 and older choose to do so.
The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery.
VR reduces or eliminates the need to use sedation or anesthesia, which carries a small but real risk of an adverse reaction. And important to parents, it eliminates the recovery time from using sedation, which shortens the visit and time missed from school and work.
A more intriguing question is whether reducing fear and anxiety in early-life experiences with the healthcare system through activities like VR will have a long-term affect on kids' attitudes toward medicine as they grow older. "If you're a screaming meemie when you come get your blood draw when you're five or seven, you're still that anxious adolescent or adult who is all quivering and sweating and avoiding healthcare," Gold says. "That's a longitudinal health outcome I'd love to get my hands on in 10-15 years from now."
Broader applications
Dermatologist Hadar Lev-Tov read about the use of VR to treat pain and decided to try it in his practice at the University of Miami Hospital. He thought, "OK, this is low risk, it's easy to do. So we got some equipment and got it done." It was so affordable he paid for it out of his own pocket, rather than wait to go through administrative channels. The results were so interesting that he decided to publish it as a series of case studies with a wide variety of patients and types of procedures.
Some of them, such as freezing off warts, are not particularly painful. "But there can be a lot of anxiety, especially for kids, which can be worse than pain and can disrupt the procedure." It can trigger a non-rational, primal fight or flight response in the limbic region of the brain.
Adults understand the need for a biopsy of a skin growth and tolerate what might be a momentary flick of pain. "But for a kid you think twice about a biopsy, both because it's a hassle and because it could be a traumatic event for a child," says Lev-Tov. VR has helped to allay such fears and improve medical care.
Integrating VR into practice has been relatively easy, primarily focusing on simple training for staff and ensuring that standard infection control practices are used in handling equipment that is used by different patients. More mundane issues are ensuring that the play back and wi-fi equipment are functioning properly. He has had a few complaints from kids when the procedure is competed and the VR is turned off prematurely, which is why he favors programs like a roller coaster ride that lasts about five minutes, ample time to take a biopsy or two.
The future is today
The pediatric neurosurgeon Auguste is collaborating with colleagues at Oakland Children's to expand use of VR into different areas of care. Cancer specialists often use a port, a bubble installed under the skin in the chest of the child, to administer chemotherapy. But the young patient's curiosity often draws their attention downward to the port and their chin can potentially contaminate or obstruct it, interfering with the procedure. So the team developed a VR game involving birds that requires players to move their heads upward, away from the port, improving administration of the drugs and reducing the risk of infection.
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor.
Other games are being developed for rehabilitation that require the use of specific nerve and muscle combinations. The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery, Auguste explains. "We can monitor their progress by the score on the game, and if it plateaus, maybe switch to another game."
Another project is trying to ease the anxiety and confusion of the patient and family experience within the hospital itself. Hospital staff are creating a personalized VR introductory walking tour that leads from the parking garage through the maze of structures and corridors in the hospital complex to Dr. Auguste's office, phlebotomy, the MRI site, and other locations they might visit. The goal is to make them familiar with key landmarks before they even set foot in the facility. "So when they come the day of the visit they have already taken that exact same path, hopefully more than once."
"They don't miss their MRI appointment and therefore they don't miss their clinical appointment with me," says Auguste. It reduces patient anxiety about the encounter and from the hospital's perspective, it will reduce costs of missed and rescheduled visits simply because patients did not go to the right place at the right time.
The VR visit will be emailed to patients ahead of time and they can watch it on a smartphone installed in a disposable cardboard viewer. Oakland Children's hopes to have the system in place by early next year. Auguste says their goal in using VR, like other health care providers across the country, is "to streamline the entire patient experience."
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor. That would be a boon to kids, parents, and the health of America.