How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next

Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”
On today’s podcast episode, Leaps.org spoke with Shai Efrati, a physician and professor in the schools of medicine and neuroscience at Tel Aviv University, about the potential health benefits of hyperbaric oxygen therapy.
On today’s podcast episode, I had a chance to speak with Shai Efrati, a physician and professor in the schools of medicine and neuroscience at Tel Aviv University. Efrati also directs the Sagol Center for Hyperbaric Medicine and Research, and our conversation in this episode focuses on the potential health benefits of hyperbaric oxygen therapy.
Efrati's studies point to a connection between the use of hyperbaric chambers and improvements for a range of health problems such as Long Covid, strokes and traumatic brain injuries. Plus, Efrati has an early line of research suggesting that hyperbaric oxygen therapy could help protect against cognitive decline in healthy people and perhaps even slow down the overall aging process.
We talk about what’s going in on the body during hyperbaric oxygen therapy that could possibly lead to transformative benefits for patients, some of whom had searched for treatments previously and come up empty. We also discuss exactly where Efrati is with this line of inquiry, both what his studies have shown and the great deal of additional research that’s needed before the healthcare system can and should fully embrace hyperbaric oxygen therapy.
Efrati and I talk about why you can’t just go on Amazon and buy something that says hyperbaric – the only way it can have a positive effect is if you access the real version of the chamber and use it correctly under the supervision of a knowledgeable physician.
I also ask Efrati what we know about the short- and long-term risks for those who follow the research-based protocol on a regular basis. And what about accessibility to people without a lot of extra cash to spend on their health? Efrati is already rolling out this therapy at a small number of specialized clinics in places like the Villages retirement community in Florida.

Shai Efrati
A vaccine for Lyme disease could be coming. But will patients accept it?
Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
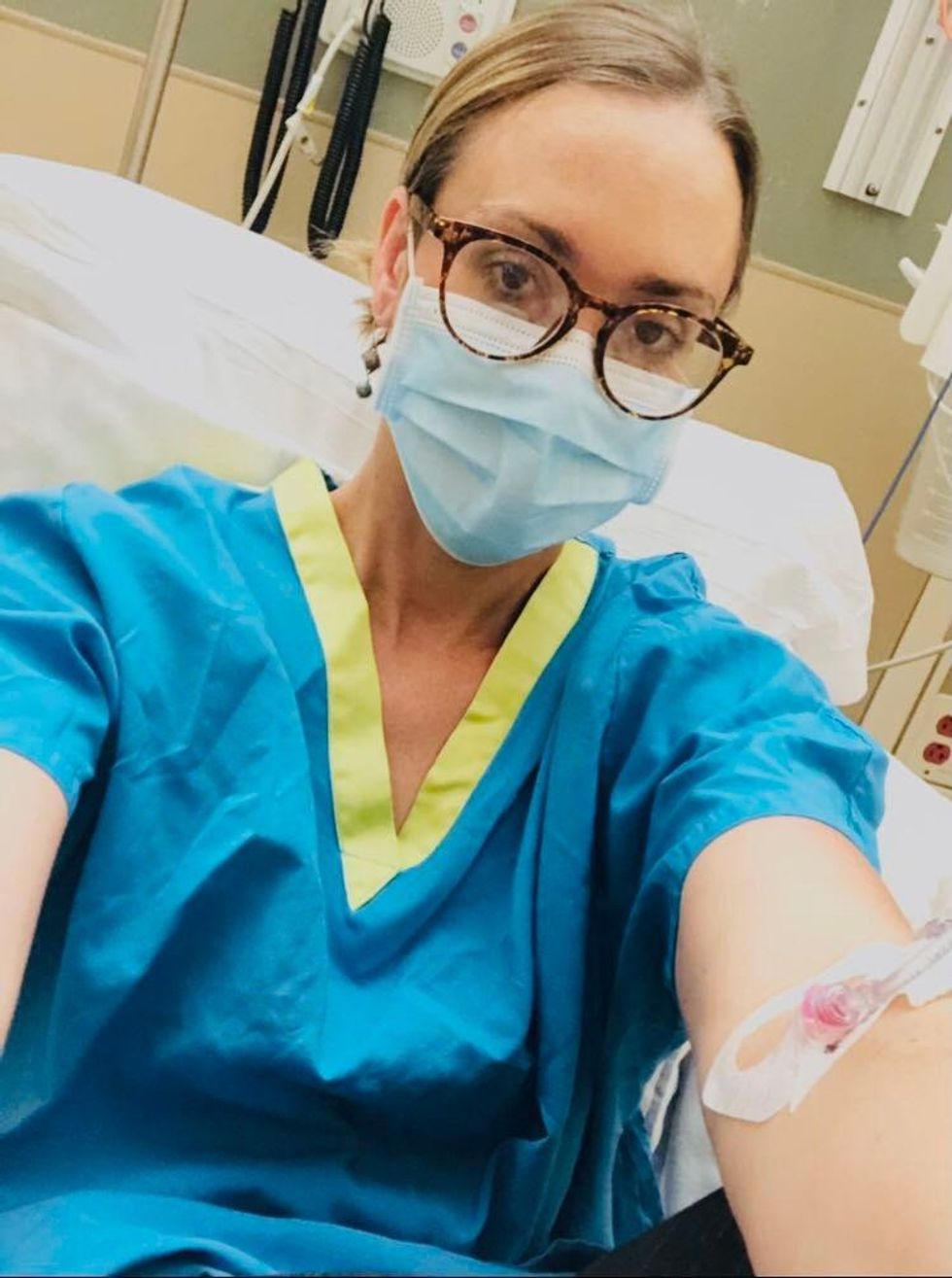
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”

