Your surgery could harm yourself and the planet. Here's what some doctors are doing about it.
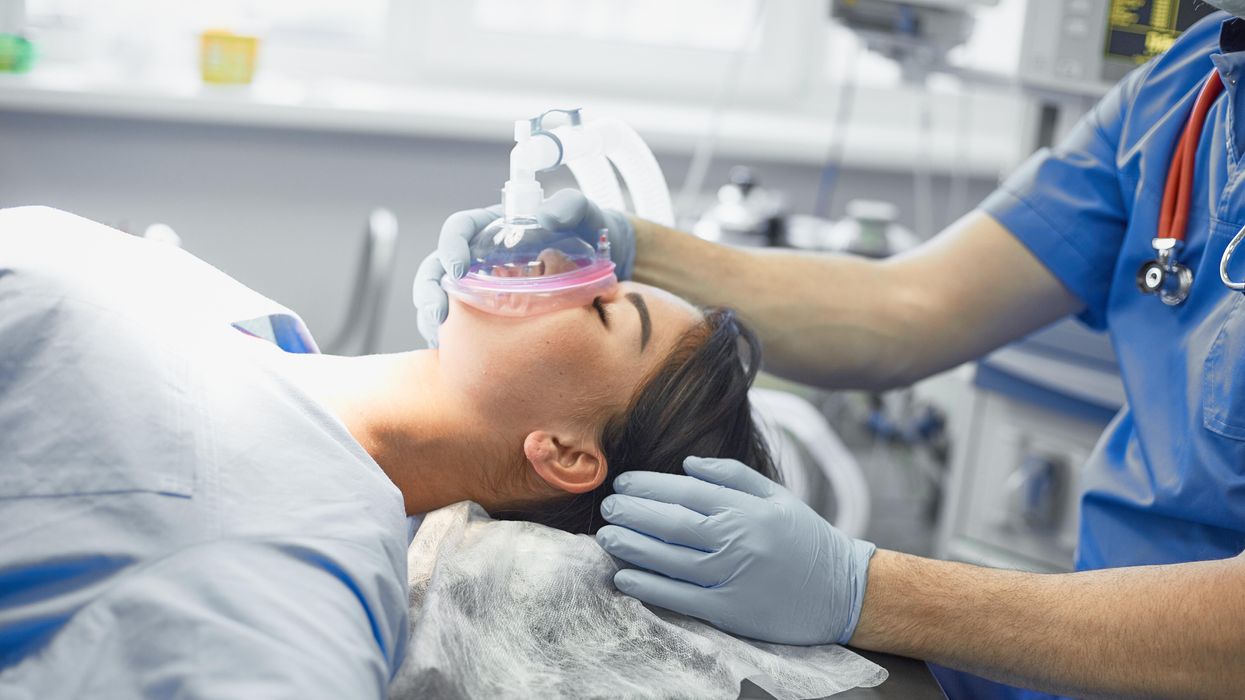
Certain gases used for anesthesia are 3,000 times more damaging for the climate than CO2. Some anesthesiologists are pointing to other solutions.
This is part 1 of a three part series on a new generation of doctors leading the charge to make the health care industry more sustainable - for the benefit of their patients and the planet. Read part 2 here and part 3 here.
Susanne Koch, an anesthesiologist and neurologist, reached a pivot point when she was up to her neck in water, almost literally. The basement of her house in Berlin had flooded in the summer of 2018, when Berlin was pummeled by unusually strong rains. After she drained the house, “I wanted to dig into facts, to understand how exactly these extreme weather events are related to climate change,” she says.
Studying the scientific literature, she realized how urgent the climate crisis is, but the biggest shock was to learn that her profession contributed substantially to the problem: Inhalation gases used during medical procedures are among the most damaging greenhouse gases. Some inhalation gases are 3,000 times more damaging for the climate than CO2, Koch discovered. “Spending seven hours in the surgery room is the equivalent of driving a car for four days nonstop,” she says. Her job of helping people at Europe’s largest university hospital, the Charité in Berlin, was inadvertently damaging both the people and the planet.
“Nobody had ever even mentioned a word about that during my training,” Koch says.
On the whole, the medical sector is responsible for a disproportionally large percentage of greenhouse gas emissions, with the U.S. as the biggest culprit. According to a key paper published in 2020 in Health Affairs, the health industry “is among the most carbon-intensive service sectors in the industrialized world,” accounting for between 4.4 percent and 4.6 percent of greenhouse gas emissions. “It’s not just anesthesia but health care that has a problem,” says Jodi Sherman, anesthesiology professor and Medical Director of the Program on Healthcare Environmental Sustainability at Yale University as well as co-director of the Lancet Planetary Health Commission on Sustainable Healthcare. In the U.S., health care greenhouse gas emissions make up about 8.5 percent of domestic greenhouse gas emissions. They rose 6 percent from 2010 to 2018, to nearly 1,700 kilograms per person, more than in any other nation.
Of course, patients worry primarily about safety, not sustainability. Yet, Koch emphasizes that “as doctors, we have the responsibility to do no harm, and this includes making sure that we use resources as sustainably as possible.” Studies show that 2018 greenhouse gas and toxic air pollutant emissions resulted in the loss of 388,000 disability-adjusted life years in the U.S. alone. “Disease burden from health care pollution is of the same order of magnitude as deaths from preventable medical errors, and should be taken just as seriously,” Sherman cautions.
When Koch, the anesthesiologist, started discussing sustainable options with colleagues, the topic was immediately met with plenty of interest. Her experience is consistent with the latest representative poll of the nonprofit Foundation Health in Germany. Nine out of ten doctors were interested in urgently finding sustainable solutions for medical services but lacked knowhow and resources. For teaching purposes, Sherman and her team have developed the Yale Gassing Greener app that allows anesthesiologists to compare how much pollution they can avoid through choosing different anesthesia methods. Sherman also published professional guidelines intended to help her colleagues better understand how various methods affect carbon emissions.
Significant traces of inhalation gases have been found in Antarctica and the Himalayas, far from the vast majority of surgery rooms.
A solution espoused by both Sherman and Koch is comparatively simple: They stopped using desflurane, which is by far the most damaging of all inhalation gases to the climate. Its greenhouse effect is 2,590 times stronger than carbon dioxide. The Yale New Haven Hospital already stopped using desflurane in 2013, becoming the first known healthcare organization to eliminate a drug based on environmental grounds. Sherman points out that this resulted in saving more than $1.2 million in costs and 1,600 tons of CO2 equivalents, about the same as the exhaust from 360 passenger vehicles per year.
At the Charité, Koch claims that switching to other anesthesiology choices, such as propofol, has eliminated 90 percent of the climate gas emissions in the anesthesiology department since 2016. Young anesthesiologists are still taught to use desflurane as the standard because desflurane is absorbed less into the patients’ bodies, and they wake up faster. However, Koch who has worked as an anesthesiologist since 2006, says that with a little bit of experience, you can learn when to stop giving the propofol so it's timed just as well with a person’s wake-up process. In addition, “patients are less likely to feel nauseous after being given propofol,” Koch says. Intravenous drugs might require more skill, she adds, "but there is nothing unique to the drug desflurane that cannot be accomplished with other medications.”
Desflurane isn’t the only gas to be concerned about. Nitrous oxide is the second most damaging because it’s extremely long-lived in the environment, and it depletes the ozone layer. Climate-conscious anesthesiologists are phasing out this gas, too, or have implemented measures to decrease leaks.
Internationally, 192 governments agreed in the Kyoto protocol of 2005 to reduce halogenated hydrocarbons – resulting from inhalation gases, including desflurane and nitrous oxide – because of their immense climate-warming potential, and in 2016, they pledged to eliminate them by 2035. However, the use of inhalation anesthetics continues to increase worldwide, not least because more people access healthcare in developing countries, and because people in industrialized countries live longer and therefore need more surgeries. Significant traces of inhalation gases have been found in Antarctica and the Himalayas, far from the vast majority of surgery rooms.
Certain companies are now pushing new technology to capture inhalation gases before they are released into the atmosphere, but both Sherman and Koch believe marketing claims of 99 percent efficiency amount to greenwashing. After investigating the technology first-hand and visiting the company that is producing such filters in Germany, Koch concluded that such technology only reduces emissions by 25 percent. And Sherman believes such initiatives are akin to the fallacy of recycling plastic. In addition to questioning their efficiency, Sherman fears such technology “gives the illusion there is a magical solution that means I don’t need to change my behavior, reduce my waste and choose less harmful options.”
Financial interests are at play, too. “Desflurane is the most expensive inhalation gas, and some think, the most expensive must be the best,” Koch says. Both Koch and Sherman lament that efforts to increase sustainability in the medical sector are entirely voluntary in their countries and led by a few dedicated individual professionals while industry-wide standards and transparency are needed, a notion expressed in the American Hospital Association’s Sustainability Roadmap.

Susanne Koch, an anesthesiologist in Berlin, wants her colleagues to stop using a gas called desflurane, which is by far the most damaging of all inhalation gases to the climate.
Adobe Stock
Other countries have done more. The European Union recommends reducing inhalation gases and even contemplated a ban of desflurane, except in medical emergencies. In 2008, the National Health Service (NHS) created a Sustainable Development Unit, which measures CO2 emissions in the U.K. health sector. NHS is the first national health service that pledged to reach net zero carbon by 2040. The carbon footprint of the NHS fell by 26 percent from 1990 to 2019, mostly due to reduced use of certain inhalers and the switch to renewable energy for heat and power. “The evidence that the climate emergency is a health emergency is overwhelming,” said Nick Watts, the NHS Chief Sustainability Officer, in a press release, “with health professionals already needing to manage its symptoms.”
Sherman is a leading voice in demanding action in the U.S. To her, comprehensive solutions start with the mandatory, transparent measurement of emissions in the health sector to tackle the biggest sources of pollution. While the Biden administration highlighted its efforts to reduce these kinds of emissions during the United Nations Climate Conference (COP27) in November 2022 and U.S. delegates announced that more than 100 health care organizations signed the voluntary Health Sector Climate Pledge, with the aim to reduce emissions by 50 percent in the next eight years, Sherman is convinced that voluntary pledges are not enough. “Voluntary measures are insufficient,” she testified in congress. “The vast majority of U.S. health care organizations remain uncommitted to timely action. Those that are committed lack policies and knowledge to support necessary changes; even worse, existing policies drive inappropriate consumption of resources and pollution.”
Both Sherman and Koch look at the larger picture. “Health care organizations have an obligation to their communities to protect public health,” Sherman says. “We must lead by example. That includes setting ambitious, science-based carbon reduction targets to achieve net zero emissions before 2050. We must quantify current emissions and their sources, particularly throughout the health care supply chains.”
In this week's Friday Five, new research that could help prevent Alzheimer's. Plus, why you should care about smart senior towns, how to reverse being drunk, money can make you happier if you're this type of person, and personalized anxiety medicine.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. Christopher Martens, director of the Delaware Center for Cogntiive Aging Research and professor of kinesiology and applied physiology at the University of Delaware, and Dr. Ilona Matysiak, visiting scholar at Iowa State University and associate professor of sociology at Maria Grzegorzewska University.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
- Could this supplement help prevent Alzheimer's?
- Why you should care about smart senior towns
- Here's how to reverse being drunk
- Money can make you happy - if you're this type of person
- Personalized anxiety medicine
As gene therapies and small molecule drugs are being studied in clinical trials, companies increasingly see the value in hiring patients to help explain the potential benefits.
As a child, Wendy Borsari participated in a health study at Boston Children’s Hospital. She was involved because heart disease and sudden cardiac arrest ran in her family as far back as seven generations. When she was 18, however, the study’s doctors told her that she had a perfectly healthy heart and didn’t have to worry.
A couple of years after graduating from college, though, the Boston native began to experience episodes of near fainting. During any sort of strenuous exercise, my blood pressure would drop instead of increasing, she recalls.
She was diagnosed at 24 with hypertrophic cardiomyopathy. Although HCM is a commonly inherited heart disease, Borsari’s case resulted from a rare gene mutation, the MYH7 gene. Her mother had been diagnosed at 27, and Borsari had already lost her grandmother and two maternal uncles to the condition. After her own diagnosis, Borsari spent most of her free time researching the disease and “figuring out how to have this condition and still be the person I wanted to be,” she says.
Then, her son was found to have the genetic mutation at birth and diagnosed with HCM at 15. Her daughter, also diagnosed at birth, later suffered five cardiac arrests.
That changed Borsari’s perspective. She decided to become a patient advocate. “I didn’t want to just be a patient with the condition,” she says. “I wanted to be more involved with the science and the biopharmaceutical industry so I could be active in helping to make it better for other patients.”
She consulted on patient advocacy for a pharmaceutical and two foundations before coming to a company called Tenaya in 2021.
“One of our core values as a company is putting patients first,” says Tenaya's CEO, Faraz Ali. “We thought of no better way to put our money where our mouth is than by bringing in somebody who is affected and whose family is affected by a genetic form of cardiomyopathy to have them make sure we’re incorporating the voice of the patient.”
Biomedical corporations and government research agencies are now incorporating patient advocacy more than ever, says Alice Lara, president and CEO of the Sudden Arrhythmia Death Syndromes Foundation in Salt Lake City, Utah. These organizations have seen the effectiveness of including patient voices to communicate and exemplify the benefits that key academic research institutions have shown in their medical studies.
“From our side of the aisle,” Lara says, “what we know as patient advocacy organizations is that educated patients do a lot better. They have a better course in their therapy and their condition, and understanding the genetics is important because all of our conditions are genetic.”
Founded in 2016, Tenaya is advancing gene therapies and small molecule drugs in clinical trials for both prevalent and rare forms of heart disease, says Ali, the CEO.
The firm's first small molecule, now in a Phase 1 clinical trial, is intended to treat heart failure with preserved ejection fraction, where the amount of blood pumped by the heart is reduced due to the heart chambers becoming weak or stiff. The condition accounts for half or more of all heart failure in the U.S., according to Ali, and is growing quickly because it's closely associated with diabetes. It’s also linked with metabolic syndrome, or a cluster of conditions including high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels.
“We have a novel molecule that is first in class and, to our knowledge, best in class to tackle that, so we’re very excited about the clinical trial,” Ali says.
The first phase of the trial is being performed with healthy participants, rather than people with the disease, to establish safety and tolerability. The researchers can also look for the drug in blood samples, which could tell them whether it's reaching its target. Ali estimates that, if the company can establish safety and that it engages the right parts of the body, it will likely begin dosing patients with the disease in 2024.
Tenaya’s therapy delivers a healthy copy of the gene so that it makes a copy of the protein missing from the patients' hearts because of their mutation. The study will start with adult patients, then pivot potentially to children and even newborns, Ali says, “where there is an even greater unmet need because the disease progresses so fast that they have no options.”
Although this work still has a long way to go, Ali is excited about the potential because the gene therapy achieved positive results in the preclinical mouse trial. This animal trial demonstrated that the treatment reduced enlarged hearts, reversed electrophysiological abnormalities, and improved the functioning of the heart by increasing the ejection fraction after the single-dose of gene therapy. That measurement remained stable to the end of the animals’ lives, roughly 18 months, Ali says.
He’s also energized by the fact that heart disease has “taken a page out of the oncology playbook” by leveraging genetic research to develop more precise and targeted drugs and gene therapies.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” says Melind Desai of the Cleveland Clinic.
Tenaya’s second program focuses on developing a gene therapy to mitigate the leading cause of hypertrophic cardiomyopathy through a specific gene called MYPBC3. The disease affects approximately 600,000 patients in the U.S. This particular genetic form, Ali explains, affects about 115,000 in the U.S. alone, so it is considered a rare disease.
“There are infants who are dying within the first weeks to months of life as a result of this mutation,” he says. “There are also adults who start having symptoms in their 20s, 30s and 40s with early morbidity and mortality.” Tenaya plans to apply before the end of this year to get the FDA’s approval to administer an investigational drug for this disease humans. If approved, the company will begin to dose patients in 2023.
“We now understand the genetics of the heart much better,” he says. “We now understand the leading genetic causes of hypertrophic myopathy, dilated cardiomyopathy and others, so that gives us the ability to take these large populations and stratify them rationally into subpopulations.”
Melind Desai, MD, who directs Cleveland Clinic’s Hypertrophic Cardiomyopathy Center, says that the goal of Tenaya’s second clinical study is to help improve the basic cardiac structure in patients with hypertrophic cardiomyopathy related to the MYPBC3 mutation.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” he says. “So this is an exciting new frontier of therapeutic investigation for MYPBC3 gene-positive patients with a chance for a cure.
Neither of Tenaya’s two therapies address the gene mutation that has affected Borsari and her family. But Ali sees opportunity down the road to develop a gene therapy for her particular gene mutation, since it is the second leading cause of cardiomyopathy. Treating the MYH7 gene is especially challenging because it requires gene editing or silencing, instead of just replacing the gene.

Wendy Borsari was diagnosed at age 24 with a commonly inherited heart disease. She joined Tenaya as a patient advocate in 2021.
Wendy Borsari
“If you add a healthy gene it will produce healthy copies,” Ali explains, “but it won’t stop the bad effects of the mutant protein the gene produces. You can only do that by silencing the gene or editing it out, which is a different, more complicated approach.”
Euan Ashley, professor of medicine and genetics at Stanford University and founding director of its Center for Inherited Cardiovascular Disease, is confident that we will see genetic therapies for heart disease within the next decade.
“We are at this really exciting moment in time where we have diseases that have been under-recognized and undervalued now being attacked by multiple companies with really modern tools,” says Ashley, author of The Genome Odyssey. “Gene therapies are unusual in the sense that they can reverse the cause of the disease, so we have the enticing possibility of actually reversing or maybe even curing these diseases.”
Although no one is doing extensive research into a gene therapy for her particular mutation yet, Borsari remains hopeful, knowing that companies such as Tenaya are moving in that direction.
“I know that’s now on the horizon,” she says. “It’s not just some pipe dream, but will happen hopefully in my lifetime or my kids’ lifetime to help them.”

