Scientists redesign bacteria to tackle the antibiotic resistance crisis
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
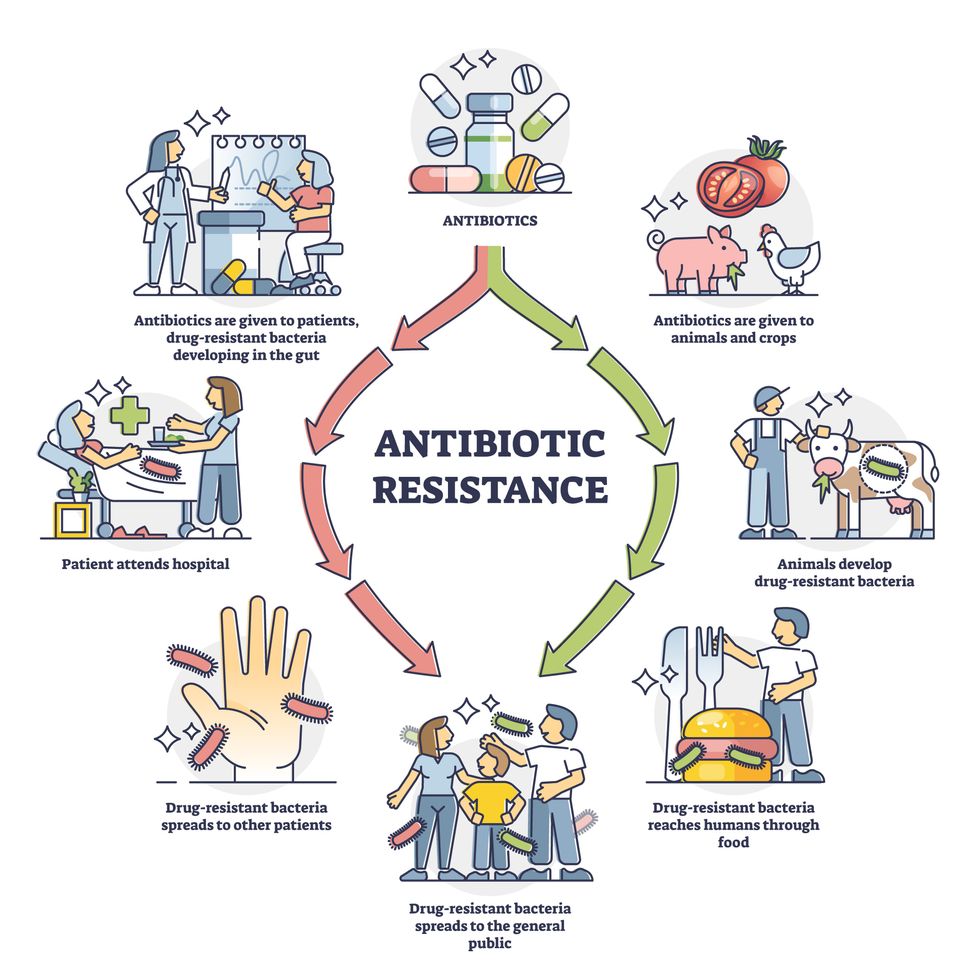
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”
How a Deadly Fire Gave Birth to Modern Medicine
The Cocoanut Grove fire in Boston in 1942 tragically claimed 490 lives, but was the catalyst for several important medical advances.
On the evening of November 28, 1942, more than 1,000 revelers from the Boston College-Holy Cross football game jammed into the Cocoanut Grove, Boston's oldest nightclub. When a spark from faulty wiring accidently ignited an artificial palm tree, the packed nightspot, which was only designed to accommodate about 500 people, was quickly engulfed in flames. In the ensuing panic, hundreds of people were trapped inside, with most exit doors locked. Bodies piled up by the only open entrance, jamming the exits, and 490 people ultimately died in the worst fire in the country in forty years.
"People couldn't get out," says Dr. Kenneth Marshall, a retired plastic surgeon in Boston and president of the Cocoanut Grove Memorial Committee. "It was a tragedy of mammoth proportions."
Within a half an hour of the start of the blaze, the Red Cross mobilized more than five hundred volunteers in what one newspaper called a "Rehearsal for Possible Blitz." The mayor of Boston imposed martial law. More than 300 victims—many of whom subsequently died--were taken to Boston City Hospital in one hour, averaging one victim every eleven seconds, while Massachusetts General Hospital admitted 114 victims in two hours. In the hospitals, 220 victims clung precariously to life, in agonizing pain from massive burns, their bodies ravaged by infection.

The scene of the fire.
Boston Public Library
Tragic Losses Prompted Revolutionary Leaps
But there is a silver lining: this horrific disaster prompted dramatic changes in safety regulations to prevent another catastrophe of this magnitude and led to the development of medical techniques that eventually saved millions of lives. It transformed burn care treatment and the use of plasma on burn victims, but most importantly, it introduced to the public a new wonder drug that revolutionized medicine, midwifed the birth of the modern pharmaceutical industry, and nearly doubled life expectancy, from 48 years at the turn of the 20th century to 78 years in the post-World War II years.
The devastating grief of the survivors also led to the first published study of post-traumatic stress disorder by pioneering psychiatrist Alexandra Adler, daughter of famed Viennese psychoanalyst Alfred Adler, who was a student of Freud. Dr. Adler studied the anxiety and depression that followed this catastrophe, according to the New York Times, and "later applied her findings to the treatment World War II veterans."
Dr. Ken Marshall is intimately familiar with the lingering psychological trauma of enduring such a disaster. His mother, an Irish immigrant and a nurse in the surgical wards at Boston City Hospital, was on duty that cold Thanksgiving weekend night, and didn't come home for four days. "For years afterward, she'd wake up screaming in the middle of the night," recalls Dr. Marshall, who was four years old at the time. "Seeing all those bodies lined up in neat rows across the City Hospital's parking lot, still in their evening clothes. It was always on her mind and memories of the horrors plagued her for the rest of her life."
The sheer magnitude of casualties prompted overwhelmed physicians to try experimental new procedures that were later successfully used to treat thousands of battlefield casualties. Instead of cutting off blisters and using dyes and tannic acid to treat burned tissues, which can harden the skin, they applied gauze coated with petroleum jelly. Doctors also refined the formula for using plasma--the fluid portion of blood and a medical technology that was just four years old--to replenish bodily liquids that evaporated because of the loss of the protective covering of skin.
"Every war has given us a new medical advance. And penicillin was the great scientific advance of World War II."
"The initial insult with burns is a loss of fluids and patients can die of shock," says Dr. Ken Marshall. "The scientific progress that was made by the two institutions revolutionized fluid management and topical management of burn care forever."
Still, they could not halt the staph infections that kill most burn victims—which prompted the first civilian use of a miracle elixir that was being secretly developed in government-sponsored labs and that ultimately ushered in a new age in therapeutics. Military officials quickly realized this disaster could provide an excellent natural laboratory to test the effectiveness of this drug and see if it could be used to treat the acute traumas of combat in this unfortunate civilian approximation of battlefield conditions. At the time, the very existence of this wondrous medicine—penicillin—was a closely guarded military secret.
From Forgotten Lab Experiment to Wonder Drug
In 1928, Alexander Fleming discovered the curative powers of penicillin, which promised to eradicate infectious pathogens that killed millions every year. But the road to mass producing enough of the highly unstable mold was littered with seemingly unsurmountable obstacles and it remained a forgotten laboratory curiosity for over a decade. But Fleming never gave up and penicillin's eventual rescue from obscurity was a landmark in scientific history.
In 1940, a group at Oxford University, funded in part by the Rockefeller Foundation, isolated enough penicillin to test it on twenty-five mice, which had been infected with lethal doses of streptococci. Its therapeutic effects were miraculous—the untreated mice died within hours, while the treated ones played merrily in their cages, undisturbed. Subsequent tests on a handful of patients, who were brought back from the brink of death, confirmed that penicillin was indeed a wonder drug. But Britain was then being ravaged by the German Luftwaffe during the Blitz, and there were simply no resources to devote to penicillin during the Nazi onslaught.
In June of 1941, two of the Oxford researchers, Howard Florey and Ernst Chain, embarked on a clandestine mission to enlist American aid. Samples of the temperamental mold were stored in their coats. By October, the Roosevelt Administration had recruited four companies—Merck, Squibb, Pfizer and Lederle—to team up in a massive, top-secret development program. Merck, which had more experience with fermentation procedures, swiftly pulled away from the pack and every milligram they produced was zealously hoarded.
After the nightclub fire, the government ordered Merck to dispatch to Boston whatever supplies of penicillin that they could spare and to refine any crude penicillin broth brewing in Merck's fermentation vats. After working in round-the-clock relays over the course of three days, on the evening of December 1st, 1942, a refrigerated truck containing thirty-two liters of injectable penicillin left Merck's Rahway, New Jersey plant. It was accompanied by a convoy of police escorts through four states before arriving in the pre-dawn hours at Massachusetts General Hospital. Dozens of people were rescued from near-certain death in the first public demonstration of the powers of the antibiotic, and the existence of penicillin could no longer be kept secret from inquisitive reporters and an exultant public. The next day, the Boston Globe called it "priceless" and Time magazine dubbed it a "wonder drug."
Within fourteen months, penicillin production escalated exponentially, churning out enough to save the lives of thousands of soldiers, including many from the Normandy invasion. And in October 1945, just weeks after the Japanese surrender ended World War II, Alexander Fleming, Howard Florey and Ernst Chain were awarded the Nobel Prize in medicine. But penicillin didn't just save lives—it helped build some of the most innovative medical and scientific companies in history, including Merck, Pfizer, Glaxo and Sandoz.
"Every war has given us a new medical advance," concludes Marshall. "And penicillin was the great scientific advance of World War II."
This Boy Struggled to Walk Before Gene Therapy. Now, Such Treatments Are Poised to Explode.
Conner Curran, now 10 years old, can walk more than two miles after gene therapy treatment for his Duchenne's muscular dystrophy.
Conner Curran was diagnosed with Duchenne's muscular dystrophy in 2015 when he was four years old. It's the most severe form of the genetic disease, with a nearly inevitable progression toward total paralysis. Many Duchenne's patients die in their teens; the average lifespan is 26.
But Conner, who is now 10, has experienced some astonishing improvements in recent years. He can now walk for more than two miles at a time – an impossible journey when he was younger.
In 2018, Conner became the very first patient to receive gene therapy specific to treating Duchenne's. In the initial clinical trial of nine children, nearly 80 percent reacted positively to the treatment). A larger-scale stage 3 clinical trial is currently underway, with initial results expected next year.
Gene therapy involves altering the genes in an individual's cells to stop or treat a disease. Such a procedure may be performed by adding new gene material to existing cells, or editing the defective genes to improve their functionality.
That the medical world is on the cusp of a successful treatment for a crippling and deadly disease is the culmination of more than 35 years of work by Dr. Jude Samulski, a professor of pharmacology at the University of North Carolina School of Medicine in Chapel Hill. More recently, he's become a leading gene therapy entrepreneur.
But Samulski likens this breakthrough to the frustrations of solving a Rubik's cube. "Just because one side is now all the color yellow does not mean that it is completely aligned," he says.
Although Conner's life and future have dramatically improved, he's not cured. The gene therapy tamed but did not extinguish his disorder: Conner is now suffering from the equivalent of Becker's muscular dystrophy, a milder form of the disease with symptoms that appear later in life and progress more slowly. Moreover, the loss of muscle cells Conner suffered prior to the treatment is permanent.
"It will take more time and more innovations," Samulski says of finding an even more effective gene therapy for muscular dystrophy.
Conner's family is still overjoyed with the results. "Jude's grit and determination gave Conner a chance at a new life, one that was not in his cards before gene therapy," says his mother Jessica Curran. She adds that "Conner is more confident than before and enjoys life, even though he has limitations, if compared to his brothers or peers."

Conner Curran holding a football post gene therapy treatment.
Courtesy of the Curran family
For now, the use of gene therapy as a treatment for diseases and disorders remains relatively isolated. On paper at least, progress appears glacially slow. In 2018, there were four FDA-approved gene therapies (excluding those reliant on bone marrow/stem cell transplants or implants). Today, there are 10. One therapy is solely for the cosmetic purpose of reducing facial lines and folds.
Nevertheless, experts in the space believe gene therapy is poised to expand dramatically.
"Certainly in the next three to five years you will see dozens of gene therapies and cell therapies be approved," says Dr. Pavan Cheruvu, who is CEO of Sio Gene Therapies in New York. The company is developing treatments for Parkinson's disease and Tay-Sachs, among other diseases.
Cheruvu's conclusion is supported by NEWDIGS, a think tank at the Massachusetts Institute of Technology that keeps tabs on gene therapy developments. NEWDIGS predicts there will be at least 60 gene therapies approved for use in the U.S. by the end of the decade. That number could be closer to 100 if Chinese researchers and biotech ventures decide the American market is a good fit for the therapies they develop.
"We are watching something of a conditional evolution, like a dot-com, or cellphones that were sizes of shoeboxes that have now matured to the size of wafers. Our space will follow along very similarly."
Dr. Carsten Brunn, a chemist by training and CEO of Selecta Biosciences outside of Boston, is developing ways to reduce the immune responses in patients who receive gene therapy. He observes that there are more than 300 therapies in development and thousands of clinical trials underway. "It's definitely an exciting time in the field," he says.
That's a far cry from the environment of little more than a decade ago. Research and investment in gene therapy had been brought low for years after the death of teenager Jesse Gelsinger in 1999 while he had been enrolled in a clinical trial to treat a liver disease. Gene therapy was a completely novel concept back then, and his death created existential questions about whether it was a proper pathway to pursue. Cheruvu, a cardiologist, calls the years after Gelsinger's death an "ice age" for gene therapy.
However, those dark years eventually yielded to a thaw. And while there have been some recent stumbles, they are considered part of the trial-and-error that has often accompanied medical research as opposed to an ominous "stop" sign.
The deaths of three patients last year receiving gene therapy for myotubular myopathy – a degenerative disease that causes severe muscle weakness – promptly ended the clinical trial in which they were enrolled. However, the incident caused few ripples beyond that. Researchers linked the deaths to dosage sizes that caused liver toxicity, as opposed to the gene therapy itself being an automatic death sentence; younger patients who received lower doses due to a less advanced disease state experienced improvements.
The gene sequencing and editing that helped create vaccines for COVID-19 in record time also bolstered the argument for more investment in research and development. Cheruvu notes that the field has usually been the domain of investors with significant expertise in the field; these days, more money is flowing in from generalists.
The Challenges Ahead
What will be the next step in gene therapy's evolution? Many of Samulski's earliest innovations came in the laboratory, for example. Then that led to him forming a company called AskBio in collaboration with the Muscular Dystrophy Association. AskBio sold its gene therapy to Pfizer five years ago to assure that enough could be manufactured for stage 3 clinical trials and eventually reach the market.
Cheruvu suggests that many future gene therapy innovations will be the result of what he calls "congruent innovation." That means publicly funded laboratories and privately funded companies might develop treatments separately or in collaboration. Or, university scientists may depend on private ventures to solve one of gene therapy's most vexing issues: producing enough finished material to test and treat on a large scale. "Manufacturing is a real bottleneck right now," Brunn says.
The alternative is referred to in the sector as the "valley of death": a lab has found a promising treatment, but is not far enough along in development to submit an investigational new drug application with the FDA. The promise withers away as a result. But the new abundance of venture capital for gene therapy has made this scenario less of an issue for private firms, some of which have received hundreds of millions of dollars in funding.
There are also numerous clinical challenges. Many gene therapies use what are known as adeno-associated virus vectors (AAVs) to deliver treatments. They are hollowed-out husks of viruses that can cause a variety of mostly mild maladies ranging from colds to pink eye. They are modified to deliver the genetic material used in the therapy. Most of these vectors trigger an antibody reaction that limits treatments to a single does or a handful of smaller ones. That can limit the potential progress for patients – an issue referred to as treatment "durability."
Although vectors from animals such as horses trigger far less of an antibody reaction in patients -- and there has been significant work done on using artificial vectors -- both are likely years away from being used on a large scale. "For the foreseeable future, AAV is the delivery system of choice," Brunn says.
Also, there will likely be demand for concurrent gene therapies that can lead to a complete cure – not only halting the progress of Duchenne's in kids like Conner Curran, but regenerating their lost muscle cells, perhaps through some form of stem cell therapy or another treatment that has yet to be devised.
Nevertheless, Samulski believes demand for imperfect treatments will be high – particularly with a disease such as muscular dystrophy, where many patients are mere months from spending the remainder of their lives in wheelchairs. But Samulski believes those therapies will also inevitably evolve into something far more effective.
"We are watching something of a conditional evolution, like a dot-com, or cellphones that were sizes of shoeboxes that have now matured to the size of wafers," he says. "Our space will follow along very similarly."
Jessica Curran will remain forever grateful for what her son has received: "Jude gave us new hope. He gave us something that is priceless – a chance to watch Conner grow up and live out his own dreams."

