Scientists redesign bacteria to tackle the antibiotic resistance crisis
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
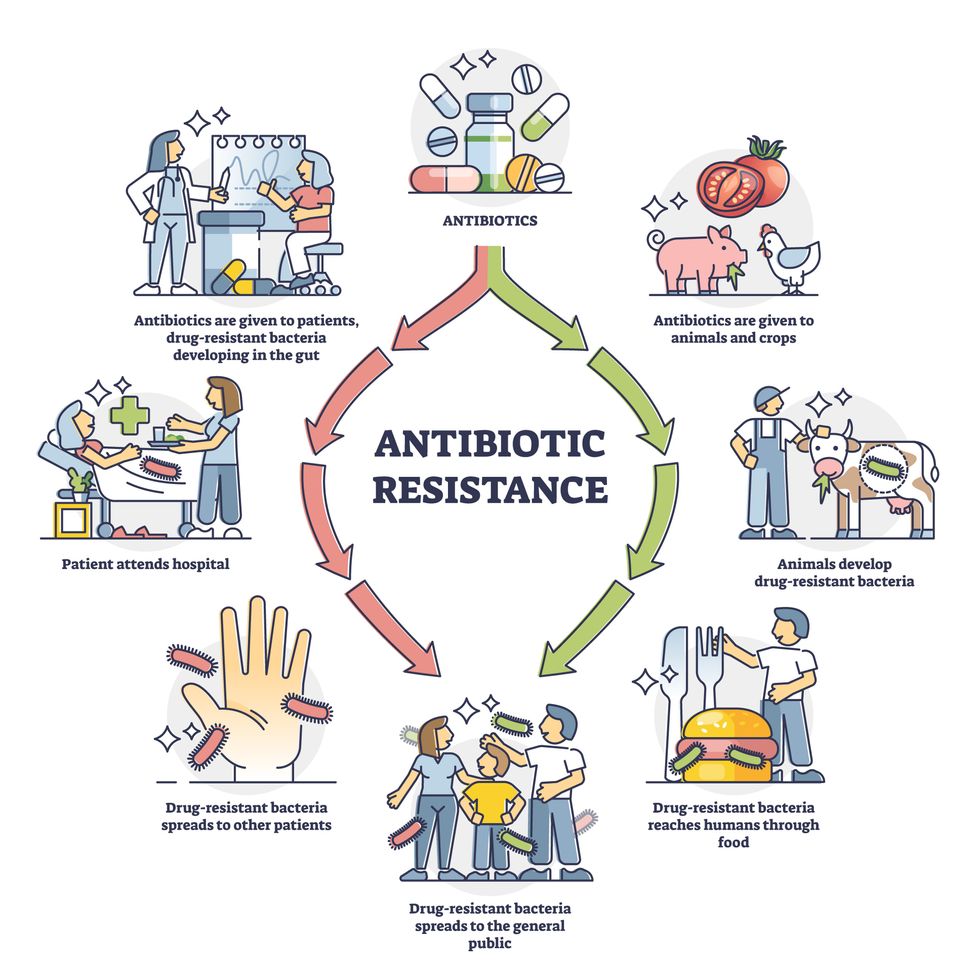
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”
When graduating college this month, many North American engineering students will take a special pledge, with a history dating back to 1925.
This spring, just like any other year, thousands of young North American engineers will graduate from their respective colleges ready to start erecting buildings, assembling machinery, and programming software, among other things. But before they take on these complex and important tasks, many of them will recite a special vow stating their ethical obligations to society, not unlike the physicians who take their Hippocratic Oath, affirming their ethos toward the patients they would treat. At the end of the ceremony, the engineers receive an iron ring, as a reminder of their promise to the millions of people their work will serve.
The ceremony isn’t just another graduation formality. As a profession, engineering has ethical weight. Moreover, engineering mistakes can be even more deadly than medical ones. A doctor’s error may cost a patient their life. But an engineering blunder may bring down a plane or crumble a building, resulting in many more fatalities. When larger projects—such as fracking, deep-sea mining or building nuclear reactors—malfunction and backfire, they can cause global disasters, afflicting millions. A vow that reminds an engineer that their work directly affects humankind and their planet is no less important than a medical oath that summons one to do no harm.
The tradition of taking an engineering oath began over a century ago in Canada. In 1922, Herbert E.T. Haultain, professor of mining engineering at the University of Toronto, presented the idea at the annual meeting of the Engineering Institute of Canada. The seven past presidents of that body were in attendance, heard Haultain’s speech and accepted his suggestion to form a committee to create an honor oath. Later, they formed the nonprofit Corporation of the Seven Wardens, which would oversee the ritual. Next year, in 1923, with the encouragement of the Seven Wardens, Haultain wrote to poet and writer Rudyard Kipling, asking him to develop a professional oath for engineers. “We are a tribe—a very important tribe within the community,” Haultain said in the letter, “but we are lacking in tribal spirit, or perhaps I should say, in manifestation of tribal spirit. Also, we are inarticulate. Can you help us?”
While Kipling is most famous now for “The Jungle Book” and perhaps his poem “Gunga Din,” he had also written a short story about engineers, “The Bridge Builders.” His poem “The Sons of Martha” can be read as a celebration of engineers:
It is their care in all the ages to take the buffet and cushion the shock.
It is their care that the gear engages; it is their care that the switches lock.
It is their care that the wheels run truly; it is their care to embark and entrain,
Tally, transport, and deliver duly the Sons of Mary by land and main.
Kipling accepted the ask and wrote the Ritual of the Calling of an Engineer, which he sent to Haultain a month later. In his response to Haultain, he stated that he preferred the word “Obligation” to “Oath.” He wrote the Obligation using Old English lettering and the old-fashioned capitalization. Kipling’s Obligation binds engineers upon their “Honor and Cold Iron” to not “suffer or pass, or be privy to the passing of, Bad Workmanship or Faulty Material,” and pardon is asked “in the presence of my betters and my equals in my Calling” for the engineer’s “assured failures and derelictions.” The hope is that when one is tempted to shoddy work by weakness or weariness, the memory of the Obligation “and the company before whom it was entered into, may return to me to aid, comfort, and restrain.”
Using the Obligation, The Seven Wardens created an induction ceremony, which seeks to unify the profession and recognize engineering’s ethics, including responsibility to the public and the need to make the best decisions possible. The induction ceremony included recitation of Kipling’s “Obligation” and incorporated an anvil, a hammer, an iron chain, and an iron ring. The inductee engineers sat inside an area marked off by the iron chain, with their more senior colleagues outside that area. At the start of the ritual, the leader beat out S-S-T in Morse code with the hammer and anvil—the letters standing for Steel, Stone, and Time. A more experienced and previously obligated engineer placed the ring on the small finger of the inductee engineer’s working hand. As per Kipling, the ring’s rough, faceted texture symbolized “the young engineer’s mind” and the difficulties engineers face in mastering their discipline.
A persistent myth purports that the original iron rings were made from the beams or bolts of the Quebec Bridge that failed twice during construction.
The first induction ceremony took place on April 25, 1925, in Montreal to obligate two of the Seven Wardens, along with four graduates from the University of Toronto class of 1893. On May 1 of that year, 14 more engineers were obligated at the University of Toronto. From that time to today most Canadian professional engineers have gone through that same ritual in their various camps, called Kipling camps—local chapters associated with various Canadian universities.
Henry Petroski, Duke University’s professor of civil engineering and history, notes in his book, “Forgive Design: Understanding Failure,” that Kipling’s poem “Sons of Martha” is often read as part of the ritual. However, sometimes inductees read Kipling’s “Hymn of Breaking Strain,” instead, which graphically depicts disastrous outcomes of engineering mistakes. The first stanza of that poem says:
The careful text-books measure
(Let all who build beware!)
The load, the shock, the pressure
Material can bear.
So, when the buckled girder
Lets down the grinding span,
'The blame of loss, or murder,
Is laid upon the man.
Not on the Stuff—the Man!
As if to strengthen the importance of these concepts, a persistent myth purports that the original iron rings were made from the beams or bolts of the Quebec Bridge that failed twice during construction. The bridge spans the St. Lawrence River upriver from Quebec City, and at the time of its construction was the world’s longest at 1,800 feet. Due to engineering errors and poor oversight, the bridge’s own weight exceeded its carrying capacity. Moreover, engineers downplayed danger when bridge beams began to warp under stress, saying that they were probably warped before they were installed. On August 29, 1907, the bridge collapsed, killing 75 of 86 workers. A second collapse occurred in 1916 when lifting equipment failed, and thirteen more workers died.
The ring myth, however, couldn’t be true. The original iron rings couldn’t have come from the failed bridge since it was made of steel, not wrought iron. Today the rings are made from stainless steel because iron deteriorates and stains engineers’ finger black.

On August 14, 2018, Morandi Bridge over Polcevera River in Genoa, Italy, collapsed from structural failure, killing 43 people.
Adobe Stock
The Seven Wardens decided to restrict the ritual to engineers trained in Canada. They copyrighted the obligation oath in Canada and the United States in 1935. Although the ritual is not a requirement for professional licensing, just like the Hippocratic Oath is not part of medical licensing, it remains a long-standing tradition.
The American Obligation of the Engineer has its own creation story, albeit a very different one. The American Order of the Engineer (OOE) was initiated in 1970, during the era of the anti-war protests, Apollo missions and the first Earth Day. On May 4, 1970, the National Guard shot into a crowd of protesters at Kent State University, killing four people. The two authors of the American obligation—Cleveland State University’s (CSU) engineering professor John Janssen and his wife Susan—reflected these historical events in the oath they wrote. Their version of the oath binds engineers to “practice integrity and fair dealing.” It also notes that their “skill carries with it the obligation to serve humanity by making the best use of the Earth’s precious wealth.” As Petroski explains in his book, “campus antiwar protestors around the country tended to view engineers as complicit in weapons proliferation [which] prompted some [CSU] engineering student leaders to look for a means of asserting some more positive values.”
Kip A. Wedel, associate professor of history and politics at Bethel College, wrote in his book, “The Obligation: A History of the Order of the Engineer,” that the ceremony was not a direct response to the Kent State shootings—it was already scheduled when the shootings happened. Yet, engineering students found the ceremony a positive action they could take in contrast to the overall turmoil. The first American ritual took place on June 4, 1970, at CSU. In total, 170 students, faculty members, and practicing engineers took the obligation. This established CSU as the first Link of the Order, as the OOE designates its local chapters. For their first ceremony, the CSU students fabricated smooth, unfaceted rings from stainless steel pipe. Later they were replaced by factory-made rings. According to Paula Ostaff, OOE’s Executive Director, about 20,000 eligible students and alumni obligate themselves yearly.
Societies hope that every engineer is imbued with a strong ethical sense and that their pledges are never far from mind. For some, the rings they wear serve a daily reminder that every paper they sign off on is touched by a physical reminder of their commitment.
These ethical and responsible engineering practices are especially salient today, when one in three American bridges needs repair or replacement, some have already collapsed, and engineers are working on projects related to the bipartisan infrastructure bill President Biden signed into law in 2021. Canada has committed $33 billion to its Investing in Canada Infrastructure Program. At the heart of these grand projects are many thousands of professional engineers, collectively working millions of hours. The professional vows they took aim to assure that the homes, bridges and airplanes they build will work as expected.
A movie still from the 1966 film "Fantastic Voyage"
In the 1966 movie "Fantastic Voyage," actress Raquel Welch and her submarine were shrunk to the size of a cell in order to eliminate a blood clot in a scientist's brain. Now, 55 years later, the scenario is becoming closer to reality.
California-based startup Bionaut Labs has developed a nanobot about the size of a grain of rice that's designed to transport medication to the exact location in the body where it's needed. If you think about it, the conventional way to deliver medicine makes little sense: A painkiller affects the entire body instead of just the arm that's hurting, and chemotherapy is flushed through all the veins instead of precisely targeting the tumor.
"Chemotherapy is delivered systemically," Bionaut-founder and CEO Michael Shpigelmacher says. "Often only a small percentage arrives at the location where it is actually needed."
But what if it was possible to send a tiny robot through the body to attack a tumor or deliver a drug at exactly the right location?
Several startups and academic institutes worldwide are working to develop such a solution but Bionaut Labs seems the furthest along in advancing its invention. "You can think of the Bionaut as a tiny screw that moves through the veins as if steered by an invisible screwdriver until it arrives at the tumor," Shpigelmacher explains. Via Zoom, he shares the screen of an X-ray machine in his Culver City lab to demonstrate how the half-transparent, yellowish device winds its way along the spine in the body. The nanobot contains a tiny but powerful magnet. The "invisible screwdriver" is an external magnetic field that rotates that magnet inside the device and gets it to move and change directions.
The current model has a diameter of less than a millimeter. Shpigelmacher's engineers could build the miniature vehicle even smaller but the current size has the advantage of being big enough to see with bare eyes. It can also deliver more medicine than a tinier version. In the Zoom demonstration, the micorobot is injected into the spine, not unlike an epidural, and pulled along the spine through an outside magnet until the Bionaut reaches the brainstem. Depending which organ it needs to reach, it could be inserted elsewhere, for instance through a catheter.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu.
Imagine moving a screw through a steak with a magnet — that's essentially how the device works. But of course, the Bionaut is considerably different from an ordinary screw: "At the right location, we give a magnetic signal, and it unloads its medicine package," Shpigelmacher says.
To start, Bionaut Labs wants to use its device to treat Parkinson's disease and brain stem gliomas, a type of cancer that largely affects children and teenagers. About 300 to 400 young people a year are diagnosed with this type of tumor. Radiation and brain surgery risk damaging sensitive brain tissue, and chemotherapy often doesn't work. Most children with these tumors live less than 18 months. A nanobot delivering targeted chemotherapy could be a gamechanger. "These patients really don't have any other hope," Shpigelmacher says.
Of course, the main challenge of the developing such a device is guaranteeing that it's safe. Because tissue is so sensitive, any mistake could risk disastrous results. In recent years, Bionaut has tested its technology in dozens of healthy sheep and pigs with no major adverse effects. Sheep make a good stand-in for humans because their brains and spines are similar to ours.

The Bionaut device is about the size of a grain of rice.
Bionaut Labs
"As the Bionaut moves through brain tissue, it creates a transient track that heals within a few weeks," Shpigelmacher says. The company is hoping to be the first to test a nanobot in humans. In December 2022, it announced that a recent round of funding drew $43.2 million, for a total of 63.2 million, enabling more research and, if all goes smoothly, human clinical trials by early next year.
Once the technique has been perfected, further applications could include addressing other kinds of brain disorders that are considered incurable now, such as Alzheimer's or Huntington's disease. "Microrobots could serve as a bridgehead, opening the gateway to the brain and facilitating precise access of deep brain structure – either to deliver medication, take cell samples or stimulate specific brain regions," Shpigelmacher says.
Robot-assisted hybrid surgery with artificial intelligence is already used in state-of-the-art surgery centers, and many medical experts believe that nanorobotics will be the instrument of the future. In 2016, three scientists were awarded the Nobel Prize in Chemistry for their development of "the world's smallest machines," nano "elevators" and minuscule motors. Since then, the scientific experiments have progressed to the point where applicable devices are moving closer to actually being implemented.
Bionaut's technology was initially developed by a research team lead by Peer Fischer, head of the independent Micro Nano and Molecular Systems Lab at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. Fischer is considered a pioneer in the research of nano systems, which he began at Harvard University more than a decade ago. He and his team are advising Bionaut Labs and have licensed their technology to the company.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu, who leads the cooperation with Bionaut Labs. He agrees with Shpigelmacher that the Bionaut's size is perfect for transporting medication loads and is researching potential applications for even smaller nanorobots, especially in the eye, where the tissue is extremely sensitive. "Nanorobots can sneak through very fine tissue without causing damage."
In "Fantastic Voyage," Raquel Welch's adventures inside the body of a dissident scientist let her swim through his veins into his brain, but her shrunken miniature submarine is attacked by antibodies; she has to flee through the nerves into the scientist's eye where she escapes into freedom on a tear drop. In reality, the exit in the lab is much more mundane. The Bionaut simply leaves the body through the same port where it entered. But apart from the dramatization, the "Fantastic Voyage" was almost prophetic, or, as Shpigelmacher says, "Science fiction becomes science reality."
This article was first published by Leaps.org on April 12, 2021.