As More People Crowdfund Medical Bills, Beware of Dubious Campaigns

Individuals seeking funding for experimental therapies may enroll in legitimate clinical trials -- or fall prey to snake oil.
Nearly a decade ago, Jamie Anderson hit his highest weight ever: 618 pounds. Depression drove him to eat and eat. He tried all kinds of diets, losing and regaining weight again and again. Then, four years ago, a friend nudged him to join a gym, and with a trainer's guidance, he embarked on a life-altering path.
Ethicists become particularly alarmed when medical crowdfunding appeals are for scientifically unfounded and potentially harmful interventions.
"The big catalyst for all of this is, I was diagnosed as a diabetic," says Anderson, a 46-year-old sales associate in the auto care department at Walmart. Within three years, he was down to 276 pounds but left with excess skin, which sagged from his belly to his mid-thighs.
Plastic surgery would cost $4,000 more than the sum his health insurance approved. That's when Anderson, who lives in Cabot, Arkansas, a suburb outside of Little Rock, turned to online crowdfunding to raise money. In a few months last year, current and former co-workers and friends of friends came up with that amount, covering the remaining expenses for the tummy tuck and overnight hospital stay.
The crowdfunding site that he used, CoFund Health, aimed to give his donors some peace of mind about where their money was going. Unlike GoFundMe and other platforms that don't restrict how donations are spent, Anderson's funds were loaded on a debit card that only worked at health care providers, so the donors "were assured that it was for medical bills only," he says.
CoFund Health was started in January 2019 in response to concerns about the legitimacy of many medical crowdfunding campaigns. As crowdfunding for health-related expenses has gained more traction on social media sites, with countless campaigns seeking to subsidize the high costs of care, it has given rise to some questionable transactions and legitimate ethical concerns.
Common examples of alleged fraud have involved misusing the donations for nonmedical purposes, feigning or embellishing the story of one's own unfortunate plight or that of another person, or impersonating someone else with an illness. Ethicists become particularly alarmed when medical crowdfunding appeals are for scientifically unfounded and potentially harmful interventions.
About 20 percent of American adults reported giving to a crowdfunding campaign for medical bills or treatments, according to a survey by AmeriSpeak Spotlight on Health from NORC, formerly called the National Opinion Research Center, a non-partisan research institution at the University of Chicago. The self-funded poll, conducted in November 2019, included 1,020 interviews with a representative sample of U.S. households. Researchers cited a 2019 City University of New York-Harvard study, which noted that medical bills are the most common basis for declaring personal bankruptcy.
Some experts contend that crowdfunding platforms should serve as gatekeepers in prohibiting campaigns for unproven treatments. Facing a dire diagnosis, individuals may go out on a limb to try anything and everything to prolong and improve the quality of their lives.
They may enroll in well-designed clinical trials, or they could fall prey "to snake oil being sold by people out there just making a buck," says Jeremy Snyder, a health sciences professor at Simon Fraser University in British Columbia, Canada, and the lead author of a December 2019 article in The Hastings Report about crowdfunding for dubious treatments.
For instance, crowdfunding campaigns have sought donations for homeopathic healing for cancer, unapproved stem cell therapy for central nervous system injury, and extended antibiotic use for chronic Lyme disease, according to an October 2018 report in the Journal of the American Medical Association.
Ford Vox, the lead author and an Atlanta-based physician specializing in brain injury, maintains that a repository should exist to monitor the outcomes of experimental treatments. "At the very least, there ought to be some tracking of what happens to the people the funds are being raised for," he says. "It would be great for an independent organization to do so."
"Even if it appears like a good cause, consumers should still do some research before donating to a crowdfunding campaign."
The Federal Trade Commission, the national consumer watchdog, cautions online that "it might be impossible for you to know if the cause is real and if the money actually gets to the intended recipient." Another caveat: Donors can't deduct contributions to individuals on tax returns.
"Even if it appears like a good cause, consumers should still do some research before donating to a crowdfunding campaign," says Malini Mithal, associate director of financial practices at the FTC. "Don't assume all medical treatments are tested and safe."
Before making any donation, it would be wise to check whether a crowdfunding site offers some sort of guarantee if a campaign ends up being fraudulent, says Kristin Judge, chief executive and founder of the Cybercrime Support Network, a Michigan-based nonprofit that serves victims before, during, and after an incident. They should know how the campaign organizer is related to the intended recipient and note whether any direct family members and friends have given funds and left supportive comments.
Donating to vetted charities offers more assurance than crowdfunding that the money will be channeled toward helping someone in need, says Daniel Billingsley, vice president of external affairs for the Oklahoma Center of Nonprofits. "Otherwise, you could be putting money into all sorts of scams." There is "zero accountability" for the crowdfunding site or the recipient to provide proof that the dollars were indeed funneled into health-related expenses.
Even if donors may have limited recourse against scammers, the "platforms have an ethical obligation to protect the people using their site from fraud," says Bryanna Moore, a postdoctoral fellow at Baylor College of Medicine's Center for Medical Ethics and Health Policy. "It's easy to take advantage of people who want to be charitable."
There are "different layers of deception" on a broad spectrum of fraud, ranging from "outright lying for a self-serving reason" to publicizing an imaginary illness to collect money genuinely needed for basic living expenses. With medical campaigns being a top category among crowdfunding appeals, it's "a lot of money that's exchanging hands," Moore says.
The advent of crowdfunding "reveals and, in some ways, reinforces a health care system that is totally broken," says Jessica Pierce, a faculty affiliate in the Center for Bioethics and Humanities at the University of Colorado Anschutz Medical Campus in Denver. "The fact that people have to scrounge for money to get life-saving treatment is unethical."
Crowdfunding also highlights socioeconomic and racial disparities by giving an unfair advantage to those who are social-media savvy and capable of crafting a compelling narrative that attracts donors. Privacy issues enter into the picture as well, because telling that narrative entails revealing personal details, Pierce says, particularly when it comes to children, "who may not be able to consent at a really informed level."
CoFund Health, the crowdfunding site on which Anderson raised the money for his plastic surgery, offers to help people write their campaigns and copy edit for proper language, says Matthew Martin, co-founder and chief executive officer. Like other crowdfunding sites, it retains a few percent of the donations for each campaign. Martin is the husband of Anderson's acquaintance from high school.
So far, the site, which is based in Raleigh, North Carolina, has hosted about 600 crowdfunding campaigns, some completed and some still in progress. Campaigns have raised as little as $300 to cover immediate dental expenses and as much as $12,000 for cancer treatments, Martin says, but most have set a goal between $5,000 and $10,000.
Whether or not someone's campaign is based on fact or fiction remains for prospective donors to decide.
The services could be cosmetic—for example, a breast enhancement or reduction, laser procedures for the eyes or skin, and chiropractic care. A number of campaigns have sought funding for transgender surgeries, which many insurers consider optional, he says.
In July 2019, a second site was hatched out of pet owners' requests for assistance with their dogs' and cats' medical expenses. Money raised on CoFund My Pet can only be used at veterinary clinics. Martin says the debit card would be declined at other merchants, just as its CoFund Health counterpart for humans will be rejected at places other than health care facilities, dental and vision providers, and pharmacies.
Whether or not someone's campaign is based on fact or fiction remains for prospective donors to decide. If a donor were to regret a transaction, he says the site would reach out to the campaign's owner but ultimately couldn't force a refund, Martin explains, because "it's hard to chase down fraud without having access to people's health records."
In some crowdfunding campaigns, the individual needs some or all the donated resources to pay for travel and lodging at faraway destinations to receive care, says Snyder, the health sciences professor and crowdfunding report author. He suggests people only give to recipients they know personally.
"That may change the calculus a little bit," tipping the decision in favor of donating, he says. As long as the treatment isn't harmful, the funds are a small gesture of support. "There's some value in that for preserving hope or just showing them that you care."
Vanessa Colimorio (left) and Sharon Kochlany (right) at a farm with their four-year-old twin daughters and one-year-old son. The kids share the same sperm donor.
Sharon Kochlany and Vanessa Colimorio's four-year-old twin girls had a classic school assignment recently: make a family tree. They drew themselves and their one-year-old brother branching off from their moms, with aunts, uncles, and grandparents forking off to the sides.
The recently-gained sovereignty of queer families stands to be lost if a consumer DNA test brings a stranger's identity out of the woodwork.
What you don't see in the invisible space between Kochlany and Colimorio, however, is the sperm donor they used to conceive all three children.
To look at a family tree like this is to see in its purest form that kinship can supersede biology—the boundaries of where this family starts and stops are clear to everyone in it, in spite of a third party's genetic involvement. This kind of self-definition has always been synonymous with LGBTQ families, especially those that rely on donor gametes (sperm or eggs) to exist.
But the world around them has changed quite suddenly: The recent consumer DNA testing boom has made it more complicated than ever for families built through reproductive technology—openly, not secretively—to maintain the strong sense of autonomy and privacy that can be crucial for their emotional security. Prospective parents and cryobanks are now mulling how best to bring a new generation of donor-conceived people into this world in a way that leaves open the choice to know more about their ancestry without obliterating an equally important choice: the right not to know about biological relatives.
For queer parents who have long fought for social acceptance, having a biological relationship to their children has been revolutionary, and using an unknown donor as a means to this end especially so. Getting help from a friend often comes with the expectation that the friend will also have social involvement in the family, which some people are comfortable with, but being able to access sperm from an unknown donor—which queer parents have only been able to openly do since the early 1980s—grants them the reproductive autonomy to create families seemingly on their own. That recently-gained sovereignty stands to be lost if a consumer DNA test brings a stranger's identity out of the woodwork.
At the same time, it's natural for donor-conceived people to want to know more about where they come from ethnically, even if they don't want to know the identity of their donor. As a donor-conceived person myself, I know my donor's self-reported ethnicity, but have often wondered how accurate it is.
Opening the Pandora's box of a consumer DNA test as a way to find out has always felt profoundly unappealing to me, however. Many people have accidentally learned they're donor-conceived by unwittingly using these tools, but I already know that about myself going in, and subsequently know I'll be connected to a large web of people whose existence I'm not interested in learning about. In addition to possibly identifying my anonymous donor, his family could also show up, along with any donor-siblings—other people with whom I share a donor. My single lesbian mom is enough for me, and the trade off to learn more about my ethnic ancestry has never seemed worth it.
In 1992, when I was born, no one was planning for how consumer DNA tests might upend or illuminate one's sense of self. But the donor community has always had to stay nimble with balancing privacy concerns and psychological well-being, so it should come as no surprise that figuring out how to do so in 2020 includes finding a way to offer ancestry insight while circumventing consumer DNA tests.
A New Paradigm
This is the rationale behind unprecedented industry news that LeapsMag can exclusively break: Within the next few weeks, California Cryobank, the largest cryobank in the country, will begin offering genetically-verified ancestry information on the free public part of every donor's anonymous profile in its database, something no other cryobanks yet offer (an exact launch date was not available at the time of publication). Currently, California Cryobank's donor profiles include a short self-reported list that might merely say, "Ancestry: German, Lebanese, Scottish."
The new information will be a report in pie chart form that details exactly what percentages of a donor's DNA come from up to 26 ethnicities—it's analogous to, but on a smaller scale than, the format offered by consumer DNA testing companies, and uses the same base technology that looks for single nucleotide polymorphisms in DNA that are associated with specific ethnicities. But crucially, because the donor takes the DNA test through California Cryobank, not a consumer-facing service, the information is not connected in a network to anyone else's DNA test. It's also taken before any offspring exist so there's no chance of revealing a donor-conceived person's identity this way.
Later, when a donor-conceived person is born, grows up, and wants information about their ethnicity from the donor side, all they need is their donor's anonymous ID number to look it up. The donor-conceived person never takes a genetic test, and therefore also can't accidentally find donor siblings this way. People who want to be connected to donor siblings can use a sibling registry where other people who want to be found share donor ID numbers and look for matches (this is something that's been available for decades, and remains so).
"With genetic testing, you have no control over who reaches out to you, and at what point in your life."
California Cryobank will require all new donors to consent to this extra level of genetic testing, setting a new standard for what information prospective parents and donor-conceived people can expect to have. In the immediate, this information will be most useful for prospective parents looking for donors with specific backgrounds, possibly ones similar to their own.
It's a solution that was actually hiding in plain sight. Two years ago, California Cryobank's partner Sema4, the company handling the genetic carrier testing that's used to screen for heritable diseases, started analyzing ethnic data in its samples. That extra information was being collected because it can help calculate a more accurate assessment of genetic risks that run in certain populations—like Ashkenazi Jews and Tay Sachs disease—than relying on oral family histories. Shortly after a plan to start collecting these extra data, Jamie Shamonki, chief medical officer of California Cryobank, realized the companies would be sitting on a goldmine for a different reason.
"I didn't want to use one of these genetic testing companies like Ancestry to accomplish this," says Shamonki. "The whole thing we're trying to accomplish is also privacy."
Consumer-facing DNA testing companies are not HIPAA compliant (whereas Sema4, which isn't direct-to-consumer, is HIPAA compliant), which means there are no legal privacy protections covering people who add their DNA to these databases. Although some companies, like 23andMe, allow users to opt-out of being connected with genetic relatives, the language can be confusing to navigate, requires a high level of knowledge and self-advocacy on the user's part, and, as an opt-out system, is not set up to protect the user from unwanted information by default; many unwittingly walk right into such information as a result.
Additionally, because consumer-facing DNA testing companies operate outside the legal purview that applies to other health care entities, like hospitals, even a person who does opt-out of being linked to genetic relatives is not protected in perpetuity from being re-identified in the future by a change in company policy. The safest option for people with privacy concerns is to stay out of these databases altogether.
For California Cryobank, the new information about donor heritage won't retroactively be added to older profiles in the system, so donor-conceived people who already exist won't benefit from the ancestry tool, but it'll be the new standard going forward. The company has about 500 available donors right now, many of which have been in their registry for a while; about 100 of those donors, all new, will have this ancestry data on their profiles.
Shamonki says it has taken about two years to get to the point of publicly including ancestry information on a donor's profile because it takes about nine months of medical and psychological screening for a donor to go from walking through the door to being added to their registry. The company wanted to wait to launch until it could offer this information for a significant number of donors. As more new donors come online under the new protocol, the number with ancestry information on their profiles will go up.
For Parents: An Unexpected Complication
While this change will no doubt be welcome progress for LGBTQ families contemplating parenthood, it'll never be possible to put this entire new order back in the box. What are such families who already have donor-conceived children losing in today's world of widespread consumer genetic testing?
Kochlany and Colimorio's twins aren't themselves much older than the moment at-home DNA testing really started to take off. They were born in 2015, and two years later the industry saw its most significant spike. By now, more than 26 million people's DNA is in databases like 23andMe and Ancestry; as a result, it's estimated that within a year, 90 percent of Americans of European descent will be identifiable through these consumer databases, by way of genetic third cousins, even if they didn't want to be found and never took the test themselves. This was the principle behind solving the Golden State Killer cold case.
The waning of privacy through consumer DNA testing fundamentally clashes with the priorities of the cyrobank industry, which has long sought to protect the privacy of donor-conceived people, even as open identification became standard. Since the 1980s, donors have been able to allow their identity to be released to any offspring who is at least 18 and wants the information. Lesbian moms pushed for this option early on so their children—who would obviously know they couldn't possibly be the biological product of both parents—would never feel cut off from the chance to know more about themselves. But importantly, the openness is not a two-way street: the donors can't ever ask for the identities of their offspring. It's the latter that consumer DNA testing really puts at stake.
"23andMe basically created the possibility that there will be donors who will have contact with their donor-conceived children, and that's not something that I think the donor community is comfortable with," says I. Glenn Cohen, director of Harvard Law School's Center for Health Law Policy, Biotechnology & Bioethics. "That's about the donor's autonomy, not the rearing parents' autonomy, or the donor-conceived child's autonomy."
Kochlany and Colimorio have an open identification donor and fully support their children reaching out to California Cryobank to get more information about him if they want to when they're 18, but having a singular name revealed isn't the same thing as having contact, nor is it the same thing as revealing a web of dozens of extended genetic relations. Their concern now is that if their kids participate in genetic testing, a stranger—someone they're careful to refer to as only "the donor" and never "dad"—will reach out to the children to begin some kind of relationship. They know other people who are contemplating giving their children DNA tests, and feel staunchly that it wouldn't be right for their family.
"With genetic testing, you have no control over who reaches out to you, and at what point in your life," Kochlany says. "[People] reaching out and trying to say, 'Hey I know who your dad is' throws a curveball. It's like, 'Wait, I never thought I had a dad.' It might put insecurities in their minds."
"We want them to have the opportunity to choose whether or not they want to reach out," Colimorio adds.
Kochlany says that when their twins are old enough to start asking questions, she and Colimorio plan to frame it like this: "The donor was kind of like a technology that helped us make you a person, and make sure that you exist," she says, role playing a conversation with their kids. "But it's not necessarily that you're looking to this person [for] support or love, or because you're missing a piece."
It's a line in the sand that's present even for couples still far off from conceiving. When Mallory Schwartz, a film and TV producer in Los Angeles, and Lauren Pietra, a marriage and family therapy associate (and Shamonki's step-daughter), talk about getting married someday, it's a package deal with talking about how they'll approach having kids. They feel there are too many variables and choices to make around family planning as a same-sex couple these days to not have those conversations simultaneously. Consumer DNA databases are already on their minds.
"It frustrates me that the DNA databases are just totally unregulated," says Schwartz. "I hope they are by the time we do this. I think everyone deserves a right to privacy when making your family [using a sperm donor]."
"I wouldn't want to create a world where people who are donor-conceived feel like they can't participate in this technology because they're trying to shut out [other] information."
On the prospect of having a donor relation pop up non-consensually for a future child, Pietra says, "I don't like it. It would be really disappointing if the child didn't want [contact], and unfortunately they're on the receiving end."
You can see how important preserving the right to keep this door closed is when you look at what's going on at The Sperm Bank of California. This pioneering cryobank was the first in the world to openly serve LGBTQ people and single women, and also the first to offer the open identification option when it opened in 1982, but not as many people are asking for their donor's identity as expected.
"We're finding a third of young people are coming forward for their donor's identity," says Alice Ruby, executive director. "We thought it would be a higher number." Viewed the other way, two-thirds of the donor-conceived people who could ethically get their donor's identity through The Sperm Bank of California are not asking the cryobank for it.
Ruby says that part of what historically made an open identification program appealing, rather than invasive or nerve-wracking, is how rigidly it's always been formatted around mutual consent, and protects against surprises for all parties. Those [donor-conceived people] who wanted more information were never barred from it, while those who wanted to remain in the dark could. No one group's wish eclipsed the other's. The potential breakdown of a system built around consent, expectations, and respect for privacy is why unregulated consumer DNA testing is most concerning to her as a path for connecting with genetic relatives.
For the last few decades in cryobanks around the world, the largest cohort of people seeking out donor sperm has been lesbian couples, followed by single women. For infertile heterosexual couples, the smallest client demographic, Ruby says donor sperm offers a solution to a medical problem, but in contrast, it historically "provided the ability for [lesbian] couples and single moms to have some reproductive autonomy." Yes, it was still a solution to a biological problem, but it was also a solution to a social one.
The Sperm Bank of California updated its registration forms to include language urging parents, donor-conceived people, and donors not to use consumer DNA tests, and to go through the cryobank if they, understandably, want to learn more about who they're connected to. But truthfully, there's not much else cryobanks can do to protect clients on any side of the donor transaction from surprise contact right now—especially not from relatives of the donor who may not even know someone in their family has donated sperm.
A Tricky Position
Personally, I've known I was donor-conceived from day one. It has never been a source of confusion, angst, or curiosity, and in fact has never loomed particularly large for me in any way. I see it merely as a type of reproductive technology—on par with in vitro fertilization—that enabled me to exist, and, now that I do exist, is irrelevant. Being confronted with my donor's identity or any donor siblings would make this fact of my conception bigger than I need it to be, as an adult with a full-blown identity derived from all of my other life experiences. But I still wonder about the minutiae of my ethnicity in much the same way as anyone else who wonders, and feel there's no safe way for me to find out without relinquishing some of my existential independence.
"People obviously want to participate in 23andMe and Ancestry because they're interested in knowing more about themselves," says Shamonki. "I wouldn't want to create a world where people who are donor-conceived feel like they can't participate in this technology because they're trying to shut out [other] information."
After all, it was the allure of that exact conceit—knowing more about oneself—that seemed to magnetically draw in millions of people to these tools in the first place. It's an experience that clearly taps into a population-wide psychic need, even—perhaps especially—if one's origins are a mystery.
As We Wait for a Vaccine, Scientists Work to Scale Up the Best COVID-19 Antibodies to Give New Patients
In this illustration of immune defense, Y-shaped proteins called antibodies attack a virus.
When we get sick, our immune system sends its soldier cells to the battlefield. Called B-cells, they "examine" the foreign particles that shouldn't be in our bloodstream—and start producing the antibodies, the proteins to neutralize the invaders.
To screen the antibodies, scientists have developed a proprietary way to make the effective ones glow – like a literal "lightbulb" moment.
The better these antibodies are at neutralizing the pathogen, the faster we recover.
The antibodies acquired from COVID-19 survivors already showed promise in treating other patients, but because they must be obtained from people, generating a regular supply is not feasible. To close the gap, researchers are trying to identify the B-cells that make the best antibodies—and then farm them in laboratories at scale.
Scientists at Berkley Lights, a biotechnology company in California, have been screening B-cells from recovered patients and testing the antibodies they release for virus-neutralizing abilities. To screen the antibodies, scientists there have developed a proprietary way to make the effective ones glow – like a literal "lightbulb" moment.
So how does it work? First, the individual B-cells are placed into microscopic chambers called nano-pens, where they secrete the antibodies. Once released, the antibodies are flushed over tiny beads that have bits of the viral particles attached to them, along with special molecules that can emit fluorescent light.
"When an antibody binds to the bead, we see a bright light on the bead," explains John Proctor, the company's senior vice president of antibody therapeutics. "So we can identify which cells are making the antibodies."
Then the antibodies are tested for their ability to counteract the coronavirus's spike proteins, which the virus uses to break into our cells. Not all antibodies are equally good at this crucial defense move—some can block only parts of the virus's machinery, while others can neutralize it completely. Proctor and his colleagues are looking for the latter.
Once scientists identify the best performing B-cells, they crack the cells open—or in scientific terms "lyse" them—and extract the genetic instructions for making the antibodies. As it turns out, B-cells aren't very efficient at pumping out massive amounts of antibodies, so scientists insert these genetic instructions into a different, more prolific type of cell.
Named Chinese Hamster Ovary Cells or CHO, these cells are commonly used in the pharmaceutical industry because they can generate therapeutic proteins en masse. Under the right nutrient conditions, which include a lot of sugar, CHO cells can keep making the antibodies at commercial levels. "They are engineered to operate in very large bioreactors," Proctor explains.
While traditional antibody screening can take three months, the Beacon System can do it in less than 24 hours.
Berkeley Lights' technology has already been used to screen the antibodies of recovered patients from Vanderbilt University Medical Center. In another example, a biotech company GenScript ProBio used the platform to screen mice engineered to have human antibodies for the coronavirus.
In addition to its small, lab-on-a-chip size, Berkeley Lights' system allows scientists to greatly speed up the screening process. While traditional antibody screening can take three months, the Beacon System can do it in less than 24 hours. "We only need one B-cell per pen and a couple of beads to see that fluorescent signal," Proctor says. "It is a more advanced way to process and analyze cells, and that level of sensitivity is unique to our technology."
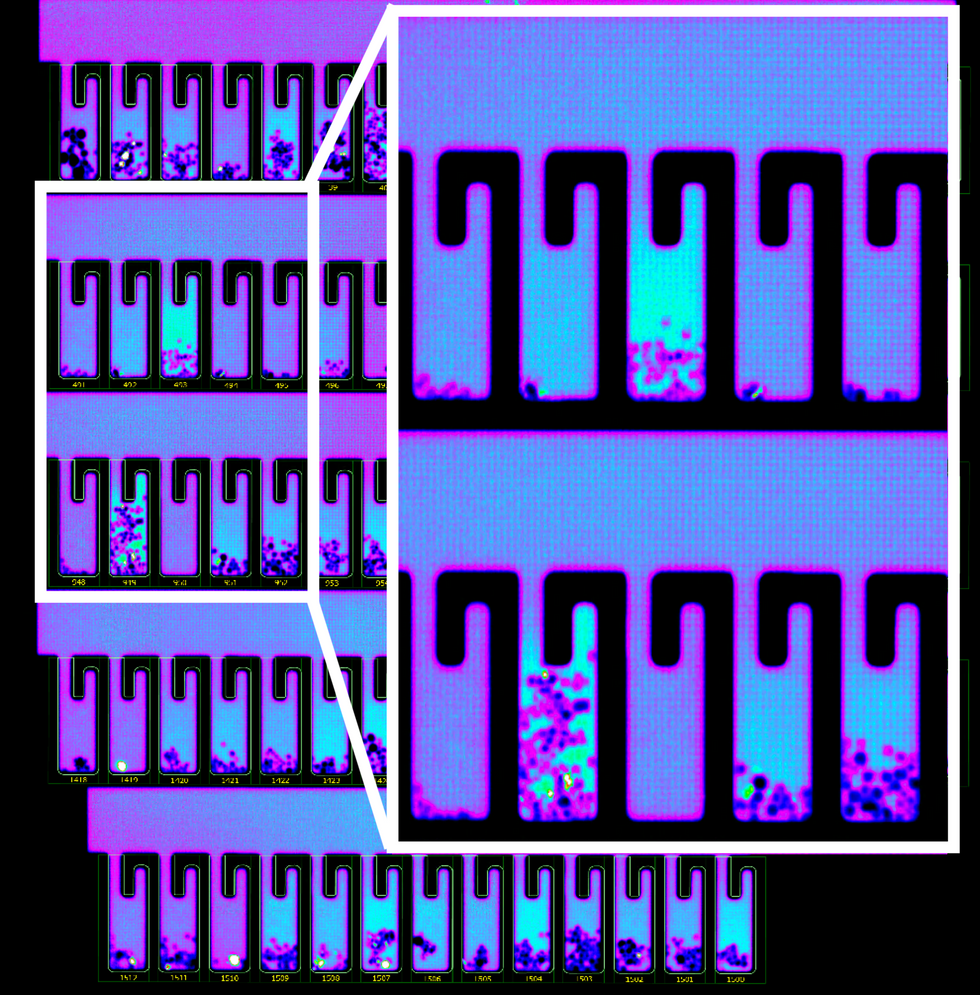
B-cells secreting antibodies inside the Berkeley Lights Beacon System Nano-Pens.
While vaccines are likely to take months to develop and test, antibodies might arrive to the battleground sooner. With the extremely limited treatment options for COVID-19, antibody-based therapeutics can potentially bridge this gap.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.