As More People Crowdfund Medical Bills, Beware of Dubious Campaigns

Individuals seeking funding for experimental therapies may enroll in legitimate clinical trials -- or fall prey to snake oil.
Nearly a decade ago, Jamie Anderson hit his highest weight ever: 618 pounds. Depression drove him to eat and eat. He tried all kinds of diets, losing and regaining weight again and again. Then, four years ago, a friend nudged him to join a gym, and with a trainer's guidance, he embarked on a life-altering path.
Ethicists become particularly alarmed when medical crowdfunding appeals are for scientifically unfounded and potentially harmful interventions.
"The big catalyst for all of this is, I was diagnosed as a diabetic," says Anderson, a 46-year-old sales associate in the auto care department at Walmart. Within three years, he was down to 276 pounds but left with excess skin, which sagged from his belly to his mid-thighs.
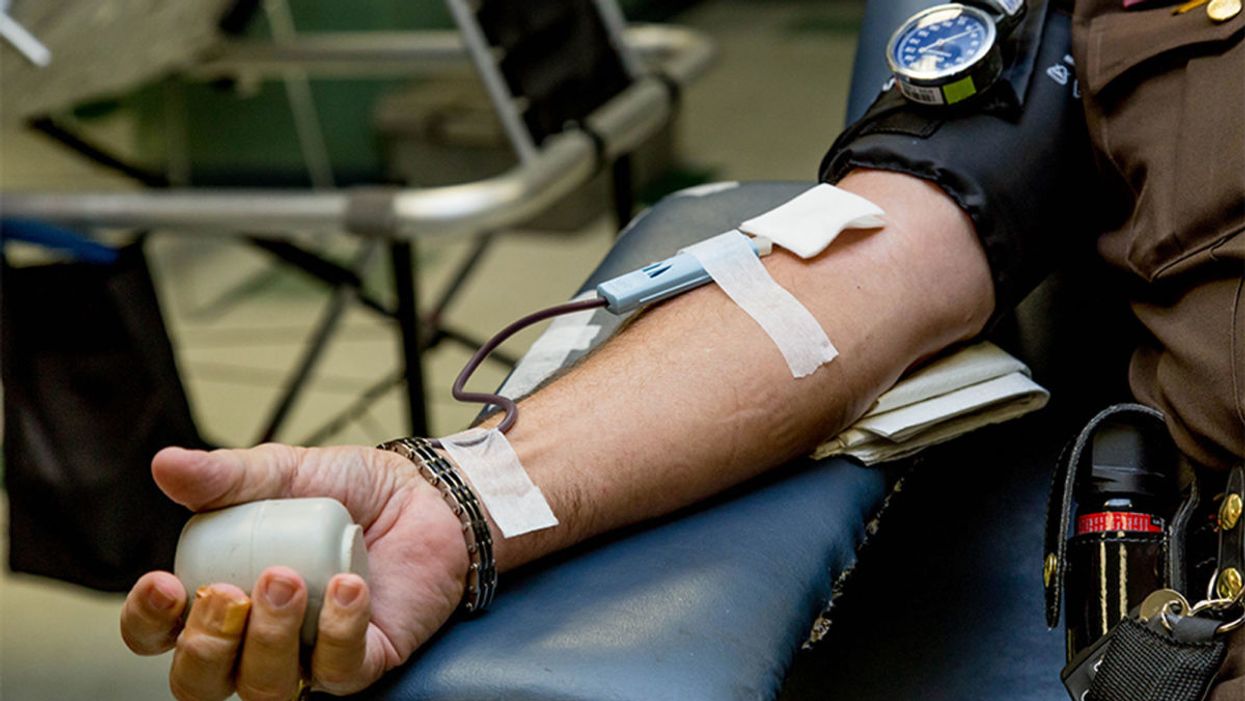
Plastic surgery would cost $4,000 more than the sum his health insurance approved. That's when Anderson, who lives in Cabot, Arkansas, a suburb outside of Little Rock, turned to online crowdfunding to raise money. In a few months last year, current and former co-workers and friends of friends came up with that amount, covering the remaining expenses for the tummy tuck and overnight hospital stay.
The crowdfunding site that he used, CoFund Health, aimed to give his donors some peace of mind about where their money was going. Unlike GoFundMe and other platforms that don't restrict how donations are spent, Anderson's funds were loaded on a debit card that only worked at health care providers, so the donors "were assured that it was for medical bills only," he says.
CoFund Health was started in January 2019 in response to concerns about the legitimacy of many medical crowdfunding campaigns. As crowdfunding for health-related expenses has gained more traction on social media sites, with countless campaigns seeking to subsidize the high costs of care, it has given rise to some questionable transactions and legitimate ethical concerns.
Common examples of alleged fraud have involved misusing the donations for nonmedical purposes, feigning or embellishing the story of one's own unfortunate plight or that of another person, or impersonating someone else with an illness. Ethicists become particularly alarmed when medical crowdfunding appeals are for scientifically unfounded and potentially harmful interventions.
About 20 percent of American adults reported giving to a crowdfunding campaign for medical bills or treatments, according to a survey by AmeriSpeak Spotlight on Health from NORC, formerly called the National Opinion Research Center, a non-partisan research institution at the University of Chicago. The self-funded poll, conducted in November 2019, included 1,020 interviews with a representative sample of U.S. households. Researchers cited a 2019 City University of New York-Harvard study, which noted that medical bills are the most common basis for declaring personal bankruptcy.
Some experts contend that crowdfunding platforms should serve as gatekeepers in prohibiting campaigns for unproven treatments. Facing a dire diagnosis, individuals may go out on a limb to try anything and everything to prolong and improve the quality of their lives.
They may enroll in well-designed clinical trials, or they could fall prey "to snake oil being sold by people out there just making a buck," says Jeremy Snyder, a health sciences professor at Simon Fraser University in British Columbia, Canada, and the lead author of a December 2019 article in The Hastings Report about crowdfunding for dubious treatments.
For instance, crowdfunding campaigns have sought donations for homeopathic healing for cancer, unapproved stem cell therapy for central nervous system injury, and extended antibiotic use for chronic Lyme disease, according to an October 2018 report in the Journal of the American Medical Association.
Ford Vox, the lead author and an Atlanta-based physician specializing in brain injury, maintains that a repository should exist to monitor the outcomes of experimental treatments. "At the very least, there ought to be some tracking of what happens to the people the funds are being raised for," he says. "It would be great for an independent organization to do so."
"Even if it appears like a good cause, consumers should still do some research before donating to a crowdfunding campaign."
The Federal Trade Commission, the national consumer watchdog, cautions online that "it might be impossible for you to know if the cause is real and if the money actually gets to the intended recipient." Another caveat: Donors can't deduct contributions to individuals on tax returns.
"Even if it appears like a good cause, consumers should still do some research before donating to a crowdfunding campaign," says Malini Mithal, associate director of financial practices at the FTC. "Don't assume all medical treatments are tested and safe."
Before making any donation, it would be wise to check whether a crowdfunding site offers some sort of guarantee if a campaign ends up being fraudulent, says Kristin Judge, chief executive and founder of the Cybercrime Support Network, a Michigan-based nonprofit that serves victims before, during, and after an incident. They should know how the campaign organizer is related to the intended recipient and note whether any direct family members and friends have given funds and left supportive comments.
Donating to vetted charities offers more assurance than crowdfunding that the money will be channeled toward helping someone in need, says Daniel Billingsley, vice president of external affairs for the Oklahoma Center of Nonprofits. "Otherwise, you could be putting money into all sorts of scams." There is "zero accountability" for the crowdfunding site or the recipient to provide proof that the dollars were indeed funneled into health-related expenses.
Even if donors may have limited recourse against scammers, the "platforms have an ethical obligation to protect the people using their site from fraud," says Bryanna Moore, a postdoctoral fellow at Baylor College of Medicine's Center for Medical Ethics and Health Policy. "It's easy to take advantage of people who want to be charitable."
There are "different layers of deception" on a broad spectrum of fraud, ranging from "outright lying for a self-serving reason" to publicizing an imaginary illness to collect money genuinely needed for basic living expenses. With medical campaigns being a top category among crowdfunding appeals, it's "a lot of money that's exchanging hands," Moore says.
The advent of crowdfunding "reveals and, in some ways, reinforces a health care system that is totally broken," says Jessica Pierce, a faculty affiliate in the Center for Bioethics and Humanities at the University of Colorado Anschutz Medical Campus in Denver. "The fact that people have to scrounge for money to get life-saving treatment is unethical."
Crowdfunding also highlights socioeconomic and racial disparities by giving an unfair advantage to those who are social-media savvy and capable of crafting a compelling narrative that attracts donors. Privacy issues enter into the picture as well, because telling that narrative entails revealing personal details, Pierce says, particularly when it comes to children, "who may not be able to consent at a really informed level."
CoFund Health, the crowdfunding site on which Anderson raised the money for his plastic surgery, offers to help people write their campaigns and copy edit for proper language, says Matthew Martin, co-founder and chief executive officer. Like other crowdfunding sites, it retains a few percent of the donations for each campaign. Martin is the husband of Anderson's acquaintance from high school.
So far, the site, which is based in Raleigh, North Carolina, has hosted about 600 crowdfunding campaigns, some completed and some still in progress. Campaigns have raised as little as $300 to cover immediate dental expenses and as much as $12,000 for cancer treatments, Martin says, but most have set a goal between $5,000 and $10,000.
Whether or not someone's campaign is based on fact or fiction remains for prospective donors to decide.
The services could be cosmetic—for example, a breast enhancement or reduction, laser procedures for the eyes or skin, and chiropractic care. A number of campaigns have sought funding for transgender surgeries, which many insurers consider optional, he says.
In July 2019, a second site was hatched out of pet owners' requests for assistance with their dogs' and cats' medical expenses. Money raised on CoFund My Pet can only be used at veterinary clinics. Martin says the debit card would be declined at other merchants, just as its CoFund Health counterpart for humans will be rejected at places other than health care facilities, dental and vision providers, and pharmacies.
Whether or not someone's campaign is based on fact or fiction remains for prospective donors to decide. If a donor were to regret a transaction, he says the site would reach out to the campaign's owner but ultimately couldn't force a refund, Martin explains, because "it's hard to chase down fraud without having access to people's health records."
In some crowdfunding campaigns, the individual needs some or all the donated resources to pay for travel and lodging at faraway destinations to receive care, says Snyder, the health sciences professor and crowdfunding report author. He suggests people only give to recipients they know personally.
"That may change the calculus a little bit," tipping the decision in favor of donating, he says. As long as the treatment isn't harmful, the funds are a small gesture of support. "There's some value in that for preserving hope or just showing them that you care."
A Single Blood Test May Soon Replace Your Annual Physical
The measurement of proteins in one blood test could provide a comprehensive snapshot of a person's health in the near future.
For all the excitement over "personalized medicine" in the last two decades, its promise has not fully come to pass. Consider your standard annual physical.
Scientists have measured thousands of proteins from a single blood test to assess many individualized health conditions at once.
Your doctor still does a blood test to check your cholesterol and gauge your risk for heart disease by considering traditional risk factors (like smoking, diabetes, blood pressure) — an evaluation that has not changed in decades.
But a high-risk number alone is not enough to tell accurately whether you will suffer from heart disease. It just reflects your risk compared to population-level averages. In other words, not every person with elevated "bad" cholesterol will have a heart attack, so how can doctors determine who truly needs to give up the cheeseburgers and who doesn't?
Now, an emerging area of research may unlock some real-time answers. For the first time, as reported in the journal Nature Medicine last week, scientists have measured thousands of proteins from a single blood test to assess many individualized health conditions at once, including liver and kidney function, diabetes risk, body fat, cardiopulmonary fitness, and even smoking and alcohol consumption. Proteins can give a clear snapshot of how your body is faring at any given moment, as well as a sneak preview at what diseases may be lurking under the surface.
"Years from now," says study co-author Peter Ganz of UCSF, "we will probably be looking back on this paper as a milestone in personalized medicine."
We spoke to Ganz about the significance of this milestone. Our interview has been edited and condensed.
Is this the first study of its kind?
Yes, it is. This is a study where we measured 5,000 proteins at once to look for patterns that could either predict the risk of future diseases or inform the current state of health. Previous to this, people have measured typically one protein at a time, and some of these individual proteins have made it into clinical practice.
An example would be a protein called C-reactive protein, which is a measure of inflammation and is used sometimes in cardiology to predict the risk of future heart attacks. But what's really new is this scale. We wanted to get away from just focusing on one problem that the patient may have at a time, whether it's heart disease or kidney disease, and by measuring a much greater number of proteins, the hope is that we could inform the health of ultimately just about every organ in the body or every tissue. It's a step forward for what I would call "a one-stop shop."
"I'm very excited about personalized medicine through proteins as opposed to genes because you get both the nature and nurture."
Three things get me excited about this. One is the convenience for the patient of a single test to determine many different diseases. The second thing is the healthcare cost savings. We estimated what the cost would be to get these 11 healthcare measures that we reported on using traditional testing and the cost was upwards of 3,000 British pounds. And even though I don't know for sure what the cost of the protein tests would ultimately be, [it could come down to about $50 to $100].
The last thing is that the measurement of proteins is part of what people have called personalized medicine or precision medicine. If you look at risk factors across the population, it may not apply to individuals. In contrast, proteins are downstream of risk factors. So proteins actually tell us whether the traditional risk factors have set in motion the necessary machinery to cause disease. Proteins are the worker bees that regulate what the human body does, and so if you can find some anomalies in the proteins, that may inform us if a disease is likely to be ongoing even in its earliest stages.
Does protein testing have advantages over genetic testing for predicting future health risks?
The problem with genomics is that genes usually don't take care of the environment. It's a blueprint, but your blueprint has no idea what you will be exposed to during your lifetime in terms of the environment and lifestyle that you may choose and medications that you may be on. These are things that proteins can account for. I'm very excited about personalized medicine through proteins as opposed to genes because you get both the nature and nurture as opposed to genomics, which only gives you nature but doesn't account for anything else.
Proteins can also be tracked over time and that's not something you can do with genes. So if your behavior improves, your genes won't change, but your proteins will.
Could this new test become a regular feature of your annual physical?
That's the idea. This would be basically almost a standalone test that you could have done every year. And hopefully you wouldn't need other tests to complement this. This could be your yearly physical.
How much more does it need to be validated before it can enter the clinic and patients can trust the results?
This was a proof-of concept study. To really make this useful, we need to expand from 11 measures of health to a hundred or more health insights, to cover the whole body. And we need to expand this to all racial groups. Three of the five centers in the study were European – all Caucasian – so it's one of our high priorities to find groups of patients with better representation of minorities.
When do you expect doctors to be routinely giving this test to patients?
Much closer to five years than 20 years. We're scaling up from 11 disease states to 100, and many of those studies are underway. Results should be done within three to five years.
Do you think insurance will cover it?
Good question. I have been approached by an insurance company that wanted to understand the product better – a major insurer, with the possibility that this could actually be cost saving.
I have to ask you a curveball -- do you think that the downfall of Theranos will make consumers hesitant to trust a new technology that relies on using a single blood sample to screen for multiple health risks?
[Laughs] You're not the first person to ask me that today. I actually got a call from Elizabeth Holmes [in 2008 when I was at Harvard]. I met with her for an afternoon and met her team two more times. I gave them advice that they completely disregarded.
In many ways, what we do is diametrically opposite to Theranos. They had a culture of secrecy, and what we do is about openness. We publish, like this paper in Nature Medicine, to show the scientific details. Our supplement is much longer than the typical academic paper. We reveal everything we know. A lot of the research we do is funded by [the National Institutes of Health], and they have strict expectations about data sharing. So we agree to make the data available on a public website. If there is something we haven't done with the data, others can do it.
So you're saying that this is not another Theranos.
No, God forbid. We hope to be the opposite.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
We Pioneered a Technology to Save Millions of Poor Children, But a Worldwide Smear Campaign Has Blocked It
On left, a picture of white rice next to Golden Rice, and on right, a girl who lost one eye due to vitamin A deficiency.
In a few weeks it will be 20 years that we three have been working together. Our project has been independently praised as one of the most influential of all projects of the last 50 years.
Two of us figured out how to make rice produce a source of vitamin A, and the rice becomes a golden color instead of white.
The project's objectives have been admired by some and vilified by others. It has directly involved teams of highly motivated people from a handful of nations, from both the private and public sector. A book, dedicated to the three of us, has been written about our work. Nevertheless, success has, so far, eluded us all. The story of our thwarted efforts is a tragedy that we hope will soon – finally – reach a milestone of potentially profound significance for humanity.
So, what have we been working on, and why haven't we succeeded yet?
Food: everybody needs it, and many are fortunate enough to have enough, even too much of it. Food is a highly emotional subject on every continent and in every culture. For a healthy life our food has to provide energy, as well as, in very small amounts, minerals and vitamins. A varied diet, easily achieved and common in industrialised countries, provides everything.
But poor people in countries where rice is grown often eat little else. White rice only provides energy: no minerals or vitamins. And the lack of one of the vitamins, vitamin A, is responsible for killing around 4,500 poor children every day. Lack of vitamin A is the biggest killer of children, and also the main cause of irreversible childhood blindness.
Our project is about fixing this one dietary deficiency – vitamin A – in this one crop – rice – for this one group of people. It is a huge group though: half of the world's population live by eating a lot of rice every day. Two of us (PB & IP) figured out how to make rice produce a source of vitamin A, and the rice becomes a golden color instead of white. The source is beta-carotene, which the human body converts to vitamin A. Beta-carotene is what makes carrots orange. Our rice is called "Golden Rice."
The technology has been donated to assist those rice eaters who suffer from vitamin A deficiency ('VAD') so that Golden Rice will cost no more than white rice, there will be no restrictions on the small farmers who grow it, and nothing extra to pay for the additional nutrition. Very small amounts of beta-carotene will contribute to alleviation of VAD, and even the earliest version of Golden Rice – which had smaller amounts than today's Golden Rice - would have helped. So far, though, no small farmer has been allowed to grow it. What happened?
To create Golden Rice, it was necessary to precisely add two genes to the 30,000 genes normally present in rice plants. One of the genes is from maize, also known as corn, and the other from a commonly eaten soil bacterium. The only difference from white rice is that Golden Rice contains beta-carotene.
It has been proven to be safe to man and the environment, and consumption of only small quantities of Golden Rice will combat VAD, with no chance of overdosing. All current Golden Rice results from one introduction of these two genes in 2004. But the use of that method – once, 15 years ago - means that Golden Rice is a 'GMO' ('genetically modified organism'). The enzymes used in the manufacture of bread, cheese, beer and wine, and the insulin which diabetics take to keep them alive, are all made from GMOs too.
The first GMO crops were created by agri-business companies. Suspicion of the technology and suspicion of commercial motivations merged, only for crop (but not enzymes or pharmaceutical) applications of GMO technology. Activists motivated by these suspicions were successful in getting the 'precautionary principle' incorporated in an international treaty which has been ratified by 166 countries and the European Union – The Cartagena Protocol.
The equivalent of 13 jumbo jets full of children crashes into the ground every day and kills them all, because of vitamin A deficiency.
This protocol is the basis of national rules governing the introduction of GMO crops in every signatory country. Government regulators in, and for, each country must agree before a GMO crop can be 'registered' to be allowed to be used by the public in that country. Currently regulatory decisions to allow Golden Rice release are being considered in Bangladesh and the Philippines.
The Cartagena Protocol obliges the regulators in each country to consider all possible risks, and to take no account of any possible benefits. Because the anti-gmo-activists' initial concerns were principally about the environment, the responsibility for governments' regulation for GMO crops – even for Golden Rice, a public health project delivered through agriculture – usually rests with the Ministry of the Environment, not the Ministry of Health or the Ministry of Agriculture.
Activists discovered, before Golden Rice was created, that inducing fear of GMO food crops from 'multinational agribusinesses' was very good for generating donations from a public that was largely illiterate about food technology and production. And this source of emotionally charged donations would cease if Golden Rice was proven to save sight and lives, because Golden Rice represented the opposite of all the tropes used in anti-GMO campaigns.
Golden Rice is created to deliver a consumer benefit, it is not for profit – to multinational agribusiness or anyone else; the technology originated in the public sector and is being delivered through the public sector. It is entirely altruistic in its motivations; which activists find impossible to accept. So, the activists believed, suspicion against Golden Rice had to be amplified, Golden Rice had to be stopped: "If we lose the Golden Rice battle, we lose the GMO war."
Activism continues to this day. And any Environment Ministry, with no responsibility for public health or agriculture, and of course an interest in avoiding controversy about its regulatory decisions, is vulnerable to such activism.
The anti-GMO crop campaigns, and especially anti-Golden Rice campaigns, have been extraordinarily effective. If so much regulation by governments is required, surely there must be something to be suspicious about: 'There is no smoke without fire'. The suspicion pervades research institutions and universities, the publishers of scientific journals and The World Health Organisation, and UNICEF: even the most scientifically literate are fearful of entanglement in activist-stoked public controversy.
The equivalent of 13 jumbo jets full of children crashes into the ground every day and kills them all, because of VAD. Yet the solution of Golden Rice, developed by national scientists in the counties where VAD is endemic, is ignored because of fear of controversy, and because poor children's deaths can be ignored without controversy.
Perhaps more controversy lies in not taking scientifically based regulatory decisions than in taking them.
The tide is turning, however. 151 Nobel Laureates, a very significant proportion of all Nobel Laureates, have called on the UN, governments of the world, and Greenpeace to cease their unfounded vilification of GMO crops in general and Golden Rice in particular. A recent Golden Rice article commented, "What shocks me is that some activists continue to misrepresent the truth about the rice. The cynic in me expects profit-driven multinationals to behave unethically, but I want to think that those voluntarily campaigning on issues they care about have higher standards."
The recently published book has exposed the frustrating saga in simple detail. And the publicity from all the above is perhaps starting to change the balance of where controversy lies. Perhaps more controversy lies in not taking scientifically based regulatory decisions than in taking them.
But until they are taken, while there continues a chance of frustrating the objectives of the Golden Rice project, the antagonism will continue. And despite a solution so close at hand, VAD-induced death and blindness, and the misery of affected families, will continue also.
© The Authors 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver applies to the data made available in this article, unless otherwise stated.