An Electrifying Idea For Roads

Companies such as Wave and Magment offer a variety of approaches for charging vehicles without plugs while they're being stored or even used, but costs stand in the way of broader adoption.
Starting this summer, the public buses in the Oberhaching suburb of Munich, Germany, won’t have to be plugged in to charge overnight anymore. Stefan Schelle, the mayor of Oberhaching, is taking advantage of the fact that an innovative startup has its offices in his community: Magment, short for “magnetizing cement,” will install its underground charging pad in the coming months. As soon as that happens, the buses will charge while they wait at the city’s main station or while stored at their overnight quarters.
In his light-filled office, Magment’s co-founder and CEO, Mauricio Esguerra, demonstrates how the new technology works: The lights on his black model car only flash when he puts the miniature Porsche directly atop the induction plate. “This works just like when you charge your iPhone on its charging pad or heat a pot on an induction range. People don’t have to be afraid of magnetic fields or anything like that,” says the 60-year-old Colombia-born entrepreneur. “The induction only gets activated when the storage battery is placed directly on top.
Patented by Esguerra, the “magnetizing concrete” is able to target the charge quite precisely. The batteries will be mounted in a box underneath the vehicles such as the retrofitted public buses. “Look, here’s one passing by,” says Esguerra, pointing out the window as a blue city bus rides past his office.
An invention finds its purpose
Esguerra grew up in Bogotá, studied physics at the Technical University Munich where he fell in love with a German woman, and started a family in her home country. For 15 years, he developed magnetic products, including the magnetizing cement, for Siemens, Europe’s largest industrial manufacturing company. The patent belonged to Siemens, of course. “But there were hardly any electric vehicles yet,” Esguerra says, “and Siemens didn’t quite know what to do with this invention.”
Esguerra changed companies a few times but, in 2015, he got an offer from Siemens. The patent for the magnetizing cement was expiring and Siemens wasn’t interested in keeping it. Would he, as the inventor, want it back? “I did not hesitate a second,” Esguerra remembers with a smile. “I’m a magnetician at heart.” That same year, he founded Magment to finally make use of the technology he created 20 years ago.
To demonstrate how his cement is made, he opens the lid of a plastic bucket filled with cement powder. Mixed in are fingernail-sized black pieces, so-called ferrites, mainly consisting of three ceramic oxides: iron, nickel and zinc. Conventionally, they are used in electronics such as cell phones, computers and cables. Molded in concrete, ferrites create a magnetic field that can transport charge to a vehicle, potentially eliminating range anxiety for EV drivers.

Molded in concrete, ferrites create a magnetic field that can transport charge to a vehicle, potentially eliminating range anxiety for EV drivers.
Magment
“Ferrites have extremely high rejection rates,” Esguerra adds. “It’s comparable to other ceramics: As soon as there is a small tear or crack, the material is rejected. We are talking about a rejection pile of 500,000 tons per year worldwide. There are mountains of unused materials.”
Exactly this fact was the starting point of his research at Siemens: “What can we do with this energy-intensive material? Back then, it was crushed up and mixed into the cement for building streets, without adding any function.” Today, too, the Magment material can simply be mixed with the conventional material and equipment of the cement industry. “We take advantage of the fact that we don’t have to build factories and don’t have high transportation costs."
In addition to saving resources, recycled ferrite also makes concrete more durable.
No plugs, no charging breaks
A young intern in the office next door winds cables around a new coil. These coils will later be lowered underground in a box, connected to the grid and encased in magnetizing concrete. The recipient box looks similar; it’s another coil but smaller, and it will be mounted underneath the carriage of the vehicle. For a car, the battery box would be 25 by 25 centimeters (about 10 inches), for a scooter five by five centimeters (about two inches).
Esguerra pushes an electric scooter into a cemented scooter rack next to his office. The charging pad is invisible. A faint beep is the only sign that it has started charging. “Childs play!” Esguerra says. “Even when someone puts in the scooter a little crooked, the charge still works. Our efficiency rate is up to 96 percent.” From this summer on, hotel chains in Munich will try out this system with their rental scooters, at a price of about 500 Euros per charging station.
Compared to plug-in charging, Magment’s benefits include smaller batteries that charge slower and, therefore, gentler, so they may last longer. Nobody needs to plug in the vehicles manually anymore. “Personally, I’ve had an EV for six years,” Esguerra says, “and how often does it happen that I forgot to plug it in overnight and then start out with a low charge in the morning? Once people get used to the invisible charging system, it will become the norm.“
There are also downsides: Most car companies aren’t ready for the new technology. Hyundai is the first carmaker that announced plans to equip some new models with inductive charging capability. “How many cars are electrified worldwide?” Esguerra asks and gives the answer himself: “One percent. And how many forklifts are electrified? More than 70 percent!” Therefore, Magment focuses on charging forklifts, e-scooters and buses.

Magment has focused most of its efforts on charging forklifts and other vehicle types that are entirely or predominantly electric, unlike cars.
Magment
On the morning of my visit to Esguerra’s office, a developer of the world’s third-biggest forklift manufacturer is there to inspect how the technology works on the ground. In the basement, a Magment engineer drives an electric forklift over a testbed with invisible charging coils, turning on the green charging light. Esguerra opens the interior of the forklift and points out the two batteries. “With our system, the forklift will only need one battery.” The savings, about 7,000 Euro per forklift, will pay for the installation of Magment’s charging system in warehouses, Esguerra calculates. “Less personnel and no unnecessary wait times for charging will lead to further savings,” he says.
To implement the new technology as efficiently as possible, Magment engineers began recording the transport routes of forklifts in warehouses. “It looks like spaghetti diagrams,” Esguerra explains. “Soon you get the areas where the forklifts pass or wait most frequently. This is where you install the chargers underground.” The forklifts will charge while in use, without having to pause for charging breaks. The method could also work for robots, for instance, in warehouses and distribution centers.
Roads of the future could be electric
Potential disadvantages might become apparent once the technology is more broadly in use. Therefore investors were initially reluctant, Esguerra admits. “Some are eager to be the first but most prefer to wait until the technology has been extensively used in real life.”
A clear hurdle today is that electrifying entire freeways with induction coils would cost at least 1 to 1.5 million Euros per kilometer. The German Department for Transportation even calculates overall costs of 14 to 47 million Euros per kilometer. So, the technology may only make sense for areas where vehicles pass or dwell the longest, like the Oberhaching train station or a busy interstate toll booth.
And yet, Magment is ramping up to compete with other companies that build larger inductive charging pads. The company just finished the first 20 meters of a testbed in Indiana, in partnership with the Purdue University and the Indiana Department of Transportation. Magment is poised to build “the world’s first contactless wireless-charging concrete pavement highway segment,” Purdue University announced.
The project, part of Purdue’s ASPIRE (Advancing Sustainability through Powered Infrastructure for Roadway Electrification) program, is financed by the National Science Foundation. “Indiana is known as the Crossroads of America, and we’re committed to fortifying our position as a transportation leader by innovating to support the emerging vehicle technology,” Governor Eric J. Holcomb said. If testing is successful, including the concrete’s capacity to charge heavy trucks operating at higher power (200 kilowatts and above), Indiana plans to identify a highway segment to install Magment’s charging pads. The earliest would be 2023 at best.
In the meantime, buses in the Californian Antelope Valley, trams at Hollywood's Universal Studios and transit buses in Tampa, Florida, are already charging with inductive technology developed by Wave, a company spun out of Utah State University. In Michigan, Governor Gretchen Whitmer announced plans to build a test route for vehicles to charge while driving, in collaboration with the Israel-based company Electreon, and this year contracted to build the first road-based charging system in the U.S. The state is providing support through an innovative grant program.
Costs remain one of the biggest obstacles, but Esguerra’s vision includes the potential that toll roads could charge a premium for inductive charging capabilities. “And in reverse, a driver who has too much energy could feed his surplus into the grid while driving,” Esguerra dreams.
Meanwhile, Wave’s upcoming big projects are moving trucks along a route in Southern California and running a UPS route between Seattle and Portland. Wave CTO Michael Masquelier describes the inductive power transfer his company champions as “similar to a tuning fork. By vibrating that fork, you sent energy through the air and it is received by another tuning fork across the room. So it’s similar to that, but it’s magnetic energy versus sound energy.”
He hopes to partner with Magment, saying that “the magnetizing cement makes installation easier and improves the energy efficiency.” More research is needed to evaluate which company’s technology will prove to be the most efficient, practical, and cost-effective.
Esguerra’s vision includes the potential that toll roads could charge a premium for inductive charging capabilities. “And in reverse, a driver who has too much energy could feed his surplus into the grid while driving,” Esguerra dreams.
The future will soon arrive in the idyllic town of Bad Staffelstein, a quaint tourist destination in the Upper Franconia region of Germany. Visitors will be taken to and from the main station and the popular thermal bath by driverless shuttles. Together with the University of Wuppertal, the regional government of Upper Franconia wants to turn its district into “the center of autonomous driving.” Magment is about to install inductive charging pads at the shuttle stations and the thermal bath, eliminating the need for the shuttles to stop for charging times. No more drivers, no cable, no range anxiety. Masquelier believes that “wireless and autonomous driving go hand in hand.” Science fiction? It will become science reality in spring 2023.
CORRECTION: An earlier version of the story erroneously mentioned that Electreon required overhead cables.
Although Jonas Salk has gone down in history for helping rid the world (almost) of polio, his revolutionary vaccine wouldn't have been possible without the world’s largest clinical trial – and the bravery of thousands of kids.
Exactly 67 years ago, in 1955, a group of scientists and reporters gathered at the University of Michigan and waited with bated breath for Dr. Thomas Francis Jr., director of the school’s Poliomyelitis Vaccine Evaluation Center, to approach the podium. The group had gathered to hear the news that seemingly everyone in the country had been anticipating for the past two years – whether the vaccine for poliomyelitis, developed by Francis’s former student Jonas Salk, was effective in preventing the disease.
Polio, at that point, had become a household name. As the highly contagious virus swept through the United States, cities closed their schools, movie theaters, swimming pools, and even churches to stop the spread. For most, polio presented as a mild illness, and was usually completely asymptomatic – but for an unlucky few, the virus took hold of the central nervous system and caused permanent paralysis of muscles in the legs, arms, and even people’s diaphragms, rendering the person unable to walk and breathe. It wasn’t uncommon to hear reports of people – mostly children – who fell sick with a flu-like virus and then, just days later, were relegated to spend the rest of their lives in an iron lung.
For two years, researchers had been testing a vaccine that would hopefully be able to stop the spread of the virus and prevent the 45,000 infections each year that were keeping the nation in a chokehold. At the podium, Francis greeted the crowd and then proceeded to change the course of human history: The vaccine, he reported, was “safe, effective, and potent.” Widespread vaccination could begin in just a few weeks. The nightmare was over.
The road to success
Jonas Salk, a medical researcher and virologist who developed the vaccine with his own research team, would rightfully go down in history as the man who eradicated polio. (Today, wild poliovirus circulates in just two countries, Afghanistan and Pakistan – with only 140 cases reported in 2020.) But many people today forget that the widespread vaccination campaign that effectively ended wild polio across the globe would have never been possible without the human clinical trials that preceded it.
As with the COVID-19 vaccine, skepticism and misinformation around the polio vaccine abounded. But even more pervasive than the skepticism was fear. The consequences of polio had arguably never been more visible.
The road to human clinical trials – and the resulting vaccine – was a long one. In 1938, President Franklin Delano Roosevelt launched the National Foundation for Infantile Paralysis in order to raise funding for research and development of a polio vaccine. (Today, we know this organization as the March of Dimes.) A polio survivor himself, Roosevelt elevated awareness and prevention into the national spotlight, even more so than it had been previously. Raising funds for a safe and effective polio vaccine became a cornerstone of his presidency – and the funds raked in by his foundation went primarily to Salk to fund his research.
The Trials Begin
Salk’s vaccine, which included an inactivated (killed) polio virus, was promising – but now the researchers needed test subjects to make global vaccination a possibility. Because the aim of the vaccine was to prevent paralytic polio, researchers decided that they had to test the vaccine in the population that was most vulnerable to paralysis – young children. And, because the rate of paralysis was so low even among children, the team required many children to collect enough data. Francis, who led the trial to evaluate Salk’s vaccine, began the process of recruiting more than one million school-aged children between the ages of six and nine in 272 counties that had the highest incidence of the disease. The participants were nicknamed the “Polio Pioneers.”
Double-blind, placebo-based trials were considered the “gold standard” of epidemiological research back in Francis's day - and they remain the best approach we have today. These rigorous scientific studies are designed with two participant groups in mind. One group, called the test group, receives the experimental treatment (such as a vaccine); the other group, called the control, receives an inactive treatment known as a placebo. The researchers then compare the effects of the active treatment against the effects of the placebo, and every researcher is “blinded” as to which participants receive what treatment. That way, the results aren’t tainted by any possible biases.
But the study was controversial in that only some of the individual field trials at the county and state levels had a placebo group. Researchers described this as a “calculated risk,” meaning that while there were risks involved in giving the vaccine to a large number of children, the bigger risk was the potential paralysis or death that could come with being infected by polio. In all, just 200,000 children across the US received a placebo treatment, while an additional 725,000 children acted as observational controls – in other words, researchers monitored them for signs of infection, but did not give them any treatment.
As with the COVID-19 vaccine, skepticism and misinformation around the polio vaccine abounded. But even more pervasive than the skepticism was fear. President Roosevelt, who had made many public and televised appearances in a wheelchair, served as a perpetual reminder of the consequences of polio, as an infection at age 39 had rendered him permanently unable to walk. The consequences of polio had arguably never been more visible, and parents signed up their children in droves to participate in the study and offer them protection.
The Polio Pioneer Legacy
In a little less than a year, roughly half a million children received a dose of Salk’s polio vaccine. While plenty of children were hesitant to get the shot, many former participants still remember the fear surrounding the disease. One former participant, a Polio Pioneer named Debbie LaCrosse, writes of her experience: “There was no discussion, no listing of pros and cons. No amount of concern over possible side effects or other unknowns associated with a new vaccine could compare to the terrifying threat of polio.” For their participation, each kid received a certificate – and sometimes a pin – with the words “Polio Pioneer” emblazoned across the front.
When Francis announced the results of the trial on April 12, 1955, people did more than just breathe a sigh of relief – they openly celebrated, ringing church bells and flooding into the streets to embrace. Salk, who had become the face of the vaccine at that point, was instantly hailed as a national hero – and teachers around the country had their students to write him ‘thank you’ notes for his years of diligent work.
But while Salk went on to win national acclaim – even accepting the Presidential Medal of Freedom for his work on the polio vaccine in 1977 – his success was due in no small part to the children (and their parents) who took a risk in order to advance medical science. And that risk paid off: By the early 1960s, the yearly cases of polio in the United States had gone down to just 910. Where before the vaccine polio had caused around 15,000 cases of paralysis each year, only ten cases of paralysis were recorded in the entire country throughout the 1970s. And in 1979, the virus that once shuttered entire towns was declared officially eradicated in this country. Thanks to the efforts of these brave pioneers, the nation – along with the majority of the world – remains free of polio even today.
Why you should (virtually) care
Virtual-first care, or V1C, could increase the quality of healthcare and make it more patient-centric by letting patients combine in-person visits with virtual options such as video for seeing their care providers.
As the pandemic turns endemic, healthcare providers have been eagerly urging patients to return to their offices to enjoy the benefits of in-person care.
But wait.
The last two years have forced all sorts of organizations to be nimble, adaptable and creative in how they work, and this includes healthcare providers’ efforts to maintain continuity of care under the most challenging of conditions. So before we go back to “business as usual,” don’t we owe it to those providers and ourselves to admit that business as usual did not work for most of the people the industry exists to help? If we’re going to embrace yet another period of change – periods that don’t happen often in our complex industry – shouldn’t we first stop and ask ourselves what we’re trying to achieve?
Certainly, COVID has shown that telehealth can be an invaluable tool, particularly for patients in rural and underserved communities that lack access to specialty care. It’s also become clear that many – though not all – healthcare encounters can be effectively conducted from afar. That said, the telehealth tactics that filled the gap during the pandemic were largely stitched together substitutes for existing visit-based workflows: with offices closed, patients scheduled video visits for help managing the side effects of their blood pressure medications or to see their endocrinologist for a quarterly check-in. Anyone whose children slogged through the last year or two of remote learning can tell you that simply virtualizing existing processes doesn’t necessarily improve the experience or the outcomes!
But what if our approach to post-pandemic healthcare came from a patient-driven perspective? We have a fleeting opportunity to advance a care model centered on convenient and equitable access that first prioritizes good outcomes, then selects approaches to care – and locations – tailored to each patient. Using the example of education, imagine how effective it would be if each student, regardless of their school district and aptitude, received such individualized attention.
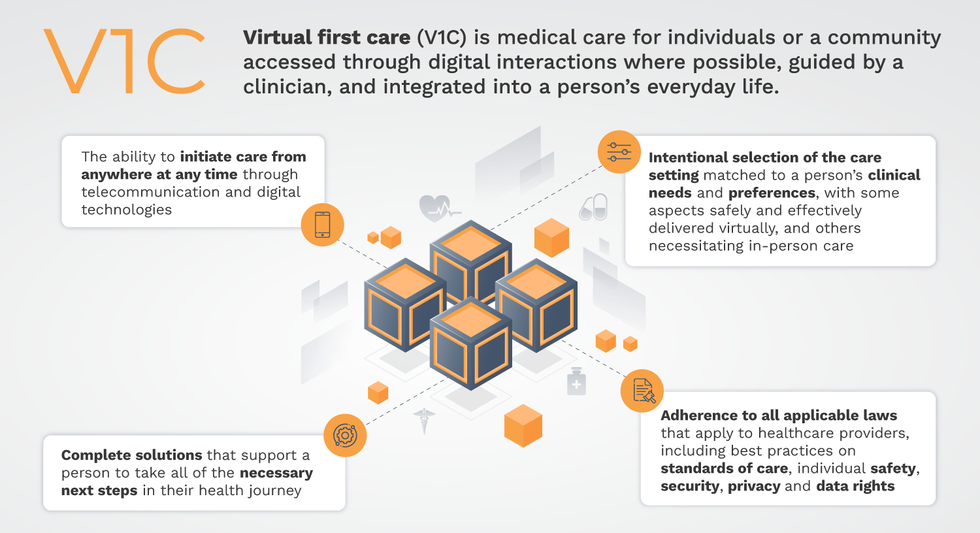
That’s the idea behind virtual-first care (V1C), a new care model centered on convenient, customized, high-quality care that integrates a full suite of services tailored directly to patients’ clinical needs and preferences. This package includes asynchronous communication such as texting; video and other live virtual modes; and in-person options.
V1C goes beyond what you might think of as standard “telehealth” by using evidence-based protocols and tools that include traditional and digital therapeutics and testing, personalized care plans, dynamic patient monitoring, and team-based approaches to care. This could include spit kits mailed for laboratory tests and complementing clinical care with health coaching. V1C also replaces some in-person exams with ongoing monitoring, using sensors for more ‘whole person’ care.
Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
Established V1C healthcare providers such as Omada, Headspace, and Heartbeat Health, as well as emerging market entrants like Oshi, Visana, and Wellinks, work with a variety of patients who have complicated long-term conditions such as diabetes, heart failure, gastrointestinal illness, endometriosis, and COPD. V1C is comprehensive in ways that are lacking in digital health and its other predecessors: it has the potential to integrate multiple data streams, incorporate more frequent touches and check-ins over time, and manage a much wider range of chronic health conditions, improving lives and reducing disease burden now and in the future.
Recognizing the pandemic-driven interest in virtual care, significant energy and resources are already flowing fast toward V1C. Some of the world’s largest innovators jumped into V1C early on: Verily, Alphabet’s Life Sciences Company, launched Onduo in 2016 to disrupt the diabetes healthcare market, and is now well positioned to scale its solutions. Major insurers like Aetna and United now offer virtual-first plans to members, responding as organizations expand virtual options for employees. Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
That was the immediate impetus for IMPACT, a consortium of V1C companies, investors, payers and patients founded last year to ensure access to high-quality, evidence-based V1C. Developed by our team at the Digital Medicine Society (DiMe) in collaboration with the American Telemedicine Association (ATA), IMPACT has begun to explore key issues that include giving patients more integrated experiences when accessing both virtual and brick-and-mortar care.
Digital Medicine Society
V1C is not, nor should it be, virtual-only care. In this new era of hybrid healthcare, success will be defined by how well providers help patients navigate the transitions. How do we smoothly hand a patient off from an onsite primary care physician to, say, a virtual cardiologist? How do we get information from a brick-and-mortar to a digital portal? How do you manage dataflow while still staying HIPAA compliant? There are many complex regulatory implications for these new models, as well as an evolving landscape in terms of privacy, security and interoperability. It will be no small task for groups like IMPACT to determine the best path forward.
None of these factors matter unless the industry can recruit and retain clinicians. Our field is facing an unprecedented workforce crisis. Traditional healthcare is making clinicians miserable, and COVID has only accelerated the trend of overworked, disenchanted healthcare workers leaving in droves. Clinicians want more interactions with patients, and fewer with computer screens – call it “More face time, less FaceTime.” No new model will succeed unless the industry can more efficiently deploy its talent – arguably its most scarce and precious resource. V1C can help with alleviating the increasing burden and frustration borne by individual physicians in today’s status quo.
In healthcare, new technological approaches inevitably provoke no shortage of skepticism. Past lessons from Silicon Valley-driven fixes have led to understandable cynicism. But V1C is a different breed of animal. By building healthcare around the patient, not the clinic, V1C can make healthcare work better for patients, payers and providers. We’re at a fork in the road: we can revert back to a broken sick-care system, or dig in and do the hard work of figuring out how this future-forward healthcare system gets financed, organized and executed. As a field, we must find the courage and summon the energy to embrace this moment, and make it a moment of change.

