An Electrifying Idea For Roads

Companies such as Wave and Magment offer a variety of approaches for charging vehicles without plugs while they're being stored or even used, but costs stand in the way of broader adoption.
Starting this summer, the public buses in the Oberhaching suburb of Munich, Germany, won’t have to be plugged in to charge overnight anymore. Stefan Schelle, the mayor of Oberhaching, is taking advantage of the fact that an innovative startup has its offices in his community: Magment, short for “magnetizing cement,” will install its underground charging pad in the coming months. As soon as that happens, the buses will charge while they wait at the city’s main station or while stored at their overnight quarters.
In his light-filled office, Magment’s co-founder and CEO, Mauricio Esguerra, demonstrates how the new technology works: The lights on his black model car only flash when he puts the miniature Porsche directly atop the induction plate. “This works just like when you charge your iPhone on its charging pad or heat a pot on an induction range. People don’t have to be afraid of magnetic fields or anything like that,” says the 60-year-old Colombia-born entrepreneur. “The induction only gets activated when the storage battery is placed directly on top.
Patented by Esguerra, the “magnetizing concrete” is able to target the charge quite precisely. The batteries will be mounted in a box underneath the vehicles such as the retrofitted public buses. “Look, here’s one passing by,” says Esguerra, pointing out the window as a blue city bus rides past his office.
An invention finds its purpose
Esguerra grew up in Bogotá, studied physics at the Technical University Munich where he fell in love with a German woman, and started a family in her home country. For 15 years, he developed magnetic products, including the magnetizing cement, for Siemens, Europe’s largest industrial manufacturing company. The patent belonged to Siemens, of course. “But there were hardly any electric vehicles yet,” Esguerra says, “and Siemens didn’t quite know what to do with this invention.”
Esguerra changed companies a few times but, in 2015, he got an offer from Siemens. The patent for the magnetizing cement was expiring and Siemens wasn’t interested in keeping it. Would he, as the inventor, want it back? “I did not hesitate a second,” Esguerra remembers with a smile. “I’m a magnetician at heart.” That same year, he founded Magment to finally make use of the technology he created 20 years ago.
To demonstrate how his cement is made, he opens the lid of a plastic bucket filled with cement powder. Mixed in are fingernail-sized black pieces, so-called ferrites, mainly consisting of three ceramic oxides: iron, nickel and zinc. Conventionally, they are used in electronics such as cell phones, computers and cables. Molded in concrete, ferrites create a magnetic field that can transport charge to a vehicle, potentially eliminating range anxiety for EV drivers.

Molded in concrete, ferrites create a magnetic field that can transport charge to a vehicle, potentially eliminating range anxiety for EV drivers.
Magment
“Ferrites have extremely high rejection rates,” Esguerra adds. “It’s comparable to other ceramics: As soon as there is a small tear or crack, the material is rejected. We are talking about a rejection pile of 500,000 tons per year worldwide. There are mountains of unused materials.”
Exactly this fact was the starting point of his research at Siemens: “What can we do with this energy-intensive material? Back then, it was crushed up and mixed into the cement for building streets, without adding any function.” Today, too, the Magment material can simply be mixed with the conventional material and equipment of the cement industry. “We take advantage of the fact that we don’t have to build factories and don’t have high transportation costs."
In addition to saving resources, recycled ferrite also makes concrete more durable.
No plugs, no charging breaks
A young intern in the office next door winds cables around a new coil. These coils will later be lowered underground in a box, connected to the grid and encased in magnetizing concrete. The recipient box looks similar; it’s another coil but smaller, and it will be mounted underneath the carriage of the vehicle. For a car, the battery box would be 25 by 25 centimeters (about 10 inches), for a scooter five by five centimeters (about two inches).
Esguerra pushes an electric scooter into a cemented scooter rack next to his office. The charging pad is invisible. A faint beep is the only sign that it has started charging. “Childs play!” Esguerra says. “Even when someone puts in the scooter a little crooked, the charge still works. Our efficiency rate is up to 96 percent.” From this summer on, hotel chains in Munich will try out this system with their rental scooters, at a price of about 500 Euros per charging station.
Compared to plug-in charging, Magment’s benefits include smaller batteries that charge slower and, therefore, gentler, so they may last longer. Nobody needs to plug in the vehicles manually anymore. “Personally, I’ve had an EV for six years,” Esguerra says, “and how often does it happen that I forgot to plug it in overnight and then start out with a low charge in the morning? Once people get used to the invisible charging system, it will become the norm.“
There are also downsides: Most car companies aren’t ready for the new technology. Hyundai is the first carmaker that announced plans to equip some new models with inductive charging capability. “How many cars are electrified worldwide?” Esguerra asks and gives the answer himself: “One percent. And how many forklifts are electrified? More than 70 percent!” Therefore, Magment focuses on charging forklifts, e-scooters and buses.

Magment has focused most of its efforts on charging forklifts and other vehicle types that are entirely or predominantly electric, unlike cars.
Magment
On the morning of my visit to Esguerra’s office, a developer of the world’s third-biggest forklift manufacturer is there to inspect how the technology works on the ground. In the basement, a Magment engineer drives an electric forklift over a testbed with invisible charging coils, turning on the green charging light. Esguerra opens the interior of the forklift and points out the two batteries. “With our system, the forklift will only need one battery.” The savings, about 7,000 Euro per forklift, will pay for the installation of Magment’s charging system in warehouses, Esguerra calculates. “Less personnel and no unnecessary wait times for charging will lead to further savings,” he says.
To implement the new technology as efficiently as possible, Magment engineers began recording the transport routes of forklifts in warehouses. “It looks like spaghetti diagrams,” Esguerra explains. “Soon you get the areas where the forklifts pass or wait most frequently. This is where you install the chargers underground.” The forklifts will charge while in use, without having to pause for charging breaks. The method could also work for robots, for instance, in warehouses and distribution centers.
Roads of the future could be electric
Potential disadvantages might become apparent once the technology is more broadly in use. Therefore investors were initially reluctant, Esguerra admits. “Some are eager to be the first but most prefer to wait until the technology has been extensively used in real life.”
A clear hurdle today is that electrifying entire freeways with induction coils would cost at least 1 to 1.5 million Euros per kilometer. The German Department for Transportation even calculates overall costs of 14 to 47 million Euros per kilometer. So, the technology may only make sense for areas where vehicles pass or dwell the longest, like the Oberhaching train station or a busy interstate toll booth.
And yet, Magment is ramping up to compete with other companies that build larger inductive charging pads. The company just finished the first 20 meters of a testbed in Indiana, in partnership with the Purdue University and the Indiana Department of Transportation. Magment is poised to build “the world’s first contactless wireless-charging concrete pavement highway segment,” Purdue University announced.
The project, part of Purdue’s ASPIRE (Advancing Sustainability through Powered Infrastructure for Roadway Electrification) program, is financed by the National Science Foundation. “Indiana is known as the Crossroads of America, and we’re committed to fortifying our position as a transportation leader by innovating to support the emerging vehicle technology,” Governor Eric J. Holcomb said. If testing is successful, including the concrete’s capacity to charge heavy trucks operating at higher power (200 kilowatts and above), Indiana plans to identify a highway segment to install Magment’s charging pads. The earliest would be 2023 at best.
In the meantime, buses in the Californian Antelope Valley, trams at Hollywood's Universal Studios and transit buses in Tampa, Florida, are already charging with inductive technology developed by Wave, a company spun out of Utah State University. In Michigan, Governor Gretchen Whitmer announced plans to build a test route for vehicles to charge while driving, in collaboration with the Israel-based company Electreon, and this year contracted to build the first road-based charging system in the U.S. The state is providing support through an innovative grant program.
Costs remain one of the biggest obstacles, but Esguerra’s vision includes the potential that toll roads could charge a premium for inductive charging capabilities. “And in reverse, a driver who has too much energy could feed his surplus into the grid while driving,” Esguerra dreams.
Meanwhile, Wave’s upcoming big projects are moving trucks along a route in Southern California and running a UPS route between Seattle and Portland. Wave CTO Michael Masquelier describes the inductive power transfer his company champions as “similar to a tuning fork. By vibrating that fork, you sent energy through the air and it is received by another tuning fork across the room. So it’s similar to that, but it’s magnetic energy versus sound energy.”
He hopes to partner with Magment, saying that “the magnetizing cement makes installation easier and improves the energy efficiency.” More research is needed to evaluate which company’s technology will prove to be the most efficient, practical, and cost-effective.
Esguerra’s vision includes the potential that toll roads could charge a premium for inductive charging capabilities. “And in reverse, a driver who has too much energy could feed his surplus into the grid while driving,” Esguerra dreams.
The future will soon arrive in the idyllic town of Bad Staffelstein, a quaint tourist destination in the Upper Franconia region of Germany. Visitors will be taken to and from the main station and the popular thermal bath by driverless shuttles. Together with the University of Wuppertal, the regional government of Upper Franconia wants to turn its district into “the center of autonomous driving.” Magment is about to install inductive charging pads at the shuttle stations and the thermal bath, eliminating the need for the shuttles to stop for charging times. No more drivers, no cable, no range anxiety. Masquelier believes that “wireless and autonomous driving go hand in hand.” Science fiction? It will become science reality in spring 2023.
CORRECTION: An earlier version of the story erroneously mentioned that Electreon required overhead cables.
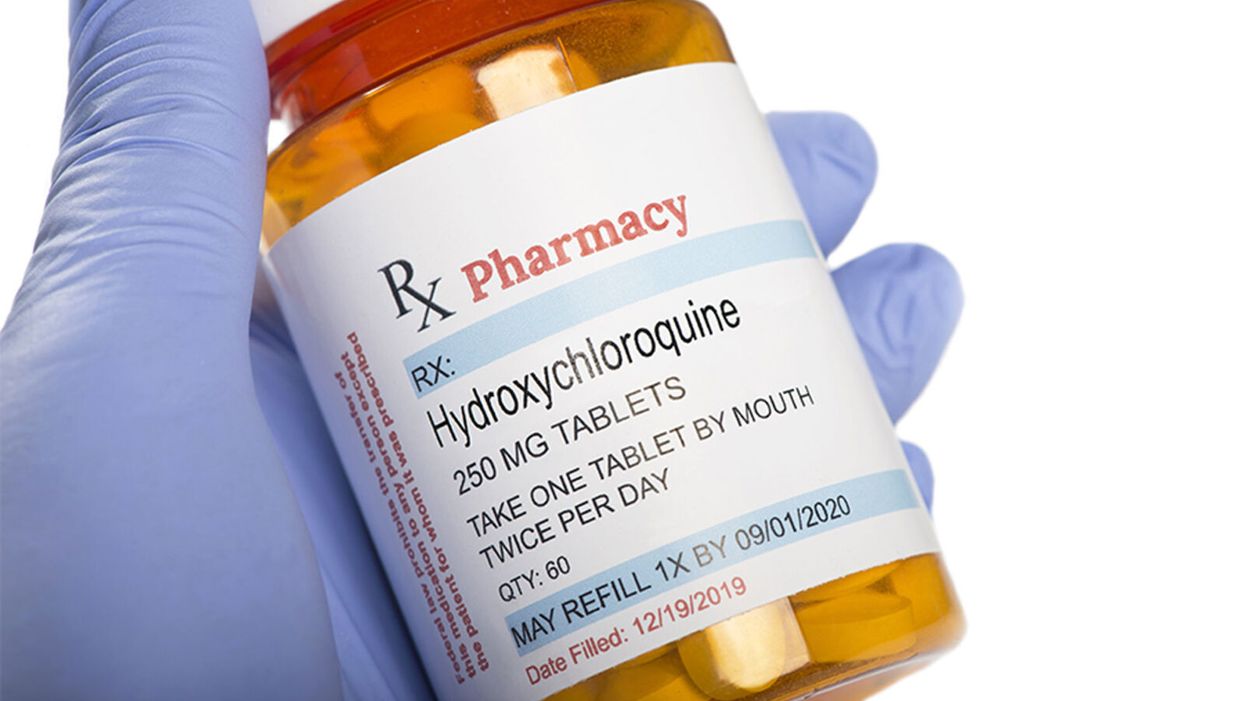
The Only Hydroxychloroquine Story You Need to Read
Hydroxychloroquine has been shown not to provide benefit for COVID-19 patients in multiple randomized controlled trials, despite continuous misinformation asserting its alleged effectiveness.
In the early days of a pandemic caused by a virus with no existing treatments, many different compounds are often considered and tried in an attempt to help patients.
It all relates back to a profound question: How do we know what we know?
Many of these treatments fall by the wayside as evidence accumulates regarding actual efficacy. At that point, other treatments become standard of care once their benefit is proven in rigorously designed trials.
However, about seven months into the pandemic, we're still seeing political resurrection of a treatment that has been systematically studied and demonstrated in well-designed randomized controlled trials to not have benefit.
The hydroxychloroquine (and by extension chloroquine) story is a complicated one that was difficult to follow even before it became infused with politics. It is a simple fact that these drugs, long approved by the Food and Drug Administration (FDA), work in Petri dishes against various viruses including coronaviruses. This set of facts provided biological plausibility to support formally studying their use in the clinical treatment and prevention of COVID-19. As evidence from these studies accumulates, it is a cognitive requirement to integrate that knowledge and not to evade it. This also means evaluating the rigor of the studies.
In recent days we have seen groups yet again promoting the use of hydroxychloroquine in, what is to me, a baffling disregard of the multiple recent studies that have shown no benefit. Indeed, though FDA-approved for other indications like autoimmune conditions and preventing malaria, the emergency use authorization for COVID-19 has been rescinded (which means the government cannot stockpile it). Still, however, many patients continue to ask for the drug, compelled by political commentary, viral videos, and anecdotal data. Yet most doctors (like myself) are refusing to write the prescriptions outside of a clinical trial – a position endorsed by professional medical organizations such as the American College of Physicians and the Infectious Diseases Society of America. Why this disconnect?
It all relates back to a profound question: How do we know what we know? In science, we use the scientific method – the process of observing reality, coming up with a hypothesis about what might be true, and testing that hypothesis as thoroughly as possible until we discover the objective truth.
The confusion we're seeing now stems from an inability to distinguish between anecdotes reported by physicians (observational data) and an actual evidence base. This is understandable among the general public but when done by a healthcare professional, it reveals a disdain for reason, logic, and the scientific method.
The Difference Between Observational Data and Randomized Controlled Trials
The power of informal observation is crucial. It is part of the scientific method but primarily as a basis for generating hypotheses that we can test. How do we conduct medical tests? The gold standard is the double-blind, randomized, placebo-controlled trial. This means that neither the researchers nor the volunteers know who is getting a drug and who is getting a sugar pill. Then both groups of the trial, called arms, can be compared to determine whether the people who got the drug fared better. This study design prevents biases and the placebo effect from confounding the data and undermining the veracity of the results.
For example, a seemingly beneficial effect might be seen in an observational study with no blinding and no control group. In such a case, all patients are openly given the drug and their doctors observe how they do. A prime example is the 36-patient single-arm study from France that generated a tremendous amount of interest after President Trump tweeted about it. But this kind of a study by its nature cannot answer the critical question: Was the positive effect because of hydroxychloroquine or just the natural course of the illness? In other words, would someone have recovered in a similar fashion regardless of the drug? What is the role of the placebo effect?
These are reasons why it is crucial to give a placebo to a control group that is as similar in every respect as possible to those receiving the intervention. Then we attempt to find out by comparing the two groups: What is the side effect profile of the drug? Are the groups large enough to detect a relatively rare safety concern? How long were the patients followed for? Was something else responsible for making the patients get better, such as the use of steroids (as likely was the case in the Henry Ford study)?
Looking at the two major hydroxychloroquine trials, it is apparent that, when studied using the best tools of clinical trials, no benefit is likely to occur.
All of these considerations amount to just a fraction of the questions that can be answered more definitively in a well-designed large randomized controlled trial than in observational studies. Indeed, an observational study from New York failed to show any benefit in hospitalized patients, showing how unclear and disparate the results can be with these types of studies. A New York retrospective study (which examined patient outcomes after they were already treated) had similar results and included the use of azithromycin.
When evaluating a study, it is also important to note whether conflicts of interest exist, as well as the quality of the peer review and the data itself. In the case of the French study, for example, the paper was published in a journal in which one of the authors was editor-in-chief, and it was accepted for publication after 24 hours. Patients who fared poorly on hydroxychloroquine were also left out of the study altogether, skewing the results.
What Randomized Controlled Trials Have Shown
Looking at the two major hydroxychloroquine trials, it is apparent that, when studied using the best tools of clinical trials, no benefit is likely to occur. The most important of these studies to announce results was part of the Recovery trial, which was designed to test multiple interventions in the treatment of COVID-19. This trial, which has yet to be formally published, was a randomized controlled trial that involved over 1500 hospitalized patients being administered hydroxychloroquine compared to over 3000 who did not receive the medication. Clinical testing requires large numbers of patients to have the power to demonstrate statistical significance -- the threshold at which any apparent benefit is more than you would expect by random chance alone.
In this study, hydroxychloroquine provided no mortality benefit or even a benefit in hospital length of stay. In fact, the opposite occurred. Hydroxychloroquine patients were more likely to stay in the hospital longer and were more likely to require mechanical ventilation. Additionally, smaller randomized trials conducted in China have not shown benefit either.
Another major study involved the use of hydroxychloroquine to prevent illness in people who were exposed to COVID-19. These results, published in The New England Journal of Medicine, included over 800 patients who were studied in a randomized double-blind controlled trial and also failed to show any benefit.
But what about adding the antibiotic azithromycin in conjunction with hydroxychloroquine? A three-arm randomized controlled study involving over 500 patients hospitalized with mild to moderate COVID-19 was conducted. Its results, also published in The New England Journal of Medicine, failed to show any benefit – with or without azithromycin – and demonstrated evidence of harm. Those who received these treatments had elevations of their liver function tests and heart rhythm abnormalities. These findings hold despite the retraction of an observational study showing similar results.
Additionally, when used in combination with remdesivir – an experimental antiviral – hydroxychloroquine has been shown to be associated with worse outcomes and more side effects.
But what about in mildly ill patients not requiring hospitalization? There was no benefit found in a randomized double-blind placebo-controlled trial of 400 patients, the majority of whom were given the drug within one day of symptoms.
Some randomized controlled studies have yet to report their findings on hydroxychloroquine in non-hospitalized patients, with the use of zinc (which has some evidence in the treatment of the common cold, another ailment that can be caused by coronaviruses). And studies have yet to come out regarding whether hydroxychloroquine can prevent people from getting sick before they are even exposed. But the preponderance of the evidence from studies designed specifically to find benefit for treating COVID-19 does not support its use outside of a research setting.
Today – even with some studies (including those with zinc) still ongoing – if a patient asked me to prescribe them hydroxychloroquine for any severity or stage of illness, with or without zinc, with or without azithromycin, I would refrain. I would explain that, based on the evidence from clinical trials that has been amassed, there is no reason to believe that it will alter the course of illness for the better.
Failing to recognize the reality of the situation runs the risk of crowding out other more promising treatments and creating animosity where none should exist.
What has been occurring is a continual shifting of goalposts with each negative hydroxychloroquine study. Those in favor of the drug protest that a trial did not include azithromycin or zinc or wasn't given at the right time to the right patients. While there may be biological plausibility to treating illness early or combining treatments with zinc, it can only be definitively shown in a randomized, controlled prospective study.
The bottom line: A study that only looks at past outcomes in one group of patients – even when well conducted – is at most hypothesis generating and cannot be used as the sole basis for a new treatment paradigm.
Some may argue that there is no time to wait for definitive studies, but no treatment is benign. The risk/benefit ratio is not the same for every possible use of the drug. For example, hydroxychloroquine has a long record of use in rheumatoid arthritis and systemic lupus (whose patients are facing shortages because of COVID-19 related demand). But the risk of side effects for many of these patients is worth taking because of the substantial benefit the drug provides in treating those conditions.
In COVID-19, however, the disease apparently causes cardiac abnormalities in a great deal of many mild cases, a situation that should prompt caution when using any drugs that have known effects on the cardiac system -- drugs like hydroxychloroquine and azithromycin.
My Own Experience
It is not the case that every physician was biased against this drug from the start. Indeed, most of us wanted it to be shown to be beneficial, as it was a generic drug that was widely available and very familiar. In fact, early in the pandemic I prescribed it to hospitalized patients on two occasions per a hospital protocol. However, it is impossible for me as a sole clinician to know whether it worked, was neutral, or was harmful. In recent days, however, I have found the hydroxychloroquine talk to have polluted the atmosphere. One recent patient was initially refusing remdesivir, a drug proven in large randomized trials to have effectiveness, because he had confused it with hydroxychloroquine.
Moving On to Other COVID Treatments: What a Treatment Should Do
The story of hydroxychloroquine illustrates a fruitless search for what we are actually looking for in a COVID-19 treatment. In short, we are looking for a medication that can decrease symptoms, decrease complications, hasten recovery, decrease hospitalizations, decrease contagiousness, decrease deaths, and prevent infection. While it is unlikely to find a single antiviral that can accomplish all of these, fulfilling even just one is important.
For example, remdesivir hastens recovery and dexamethasone decreases mortality. Definitive results of the use of convalescent plasma and immunomodulating drugs such as siltuxamab, baricitinib, and anakinra (for use in the cytokine storms characteristic of severe disease) are still pending, as are the trials with monoclonal antibodies.
While it was crucial that the medical and scientific community definitively answer the questions surrounding the use of chloroquine and hydroxychloroquine in the treatment of COVID-19, it is time to face the facts and accept that its use for the treatment of this disease is not likely to be beneficial. Failing to recognize the reality of the situation runs the risk of crowding out other more promising treatments and creating animosity where none should exist.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
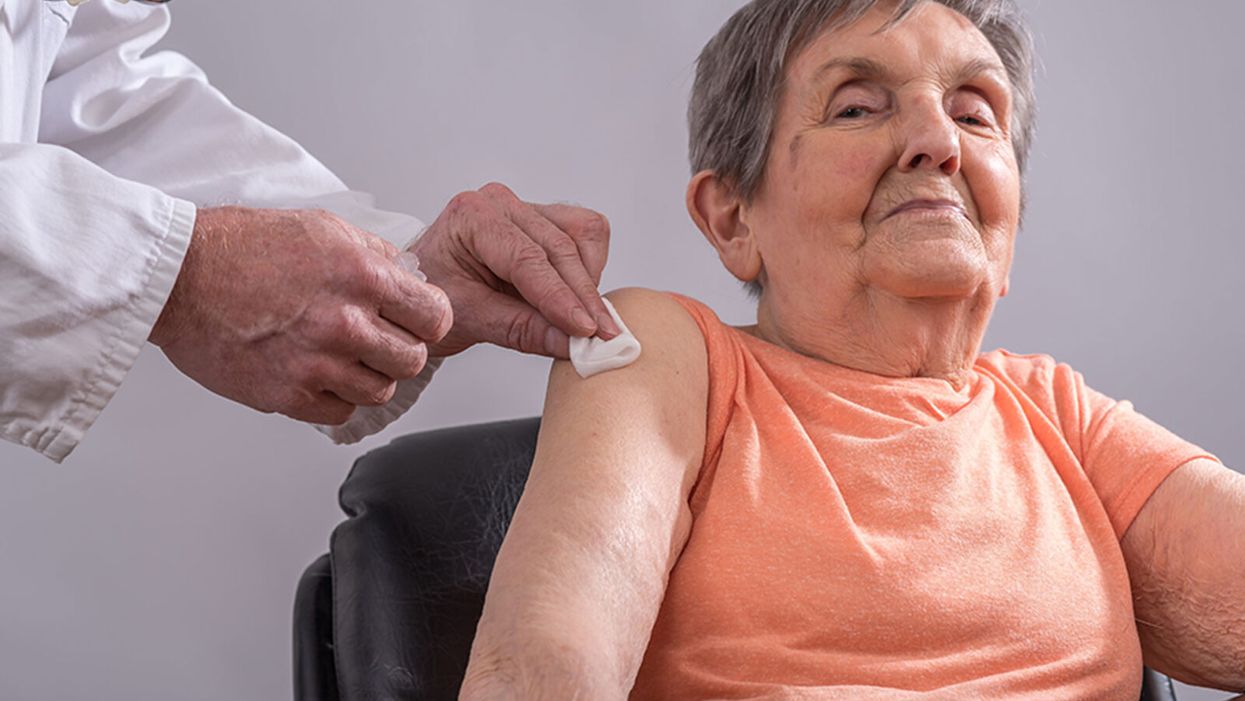
Drugs That Trick Older People’s Bodies to Behave Younger Might Boost the Effectiveness of a COVID-19 Vaccine
Because vaccination may be less effective in older people, it is imperative to test now whether immune boosters could help the efficacy of a COVID-19 vaccine in the elderly.
In our April 23rd editorial for this magazine, we argued that addressing the COVID-19 pandemic requires that we both fight the SARS-CoV-2 virus and fortify the human hosts who are most vulnerable to it.
Two recent phase 2 studies in older adults have suggested that a new category of drugs called rapalogues can in some cases increase the immunization capacity of older adults.
Because people over 70 account for more than 80 percent of reported COVID-19 deaths globally, this means we must do everything possible to protect our elders.
A range of recent studies have suggested that systemic knobs might metaphorically be turned to slow the cellular aging process, making us better able to fight off the many diseases correlated with aging. These types of systemic changes might be used to stem the specific decline in immunity caused by aging and to increases the biological capacity of elderly people to effectively fight viral infection.
But while helping make older people more resilient in the face of a viral infection is critical, that's not the only way geroscience can help in our fight against this deadly pandemic.
As we move toward hopefully developing one or more COVID-19 vaccines, researchers must more fully appreciate the ways in which traditional vaccines can be less effective in older people than in younger ones.
Repeated studies have shown that the flu vaccine, for example, has lower efficacy in older people than in younger ones. Older people tend to develop fewer antibodies after being vaccinated because a subset of their white blood cells, called T cells, have become less responsive over time. Some inflammatory peptides that increase with aging are also preventing the action of those T cells.
This is why there's a distinct possibility that a future COVD-19 vaccine, particularly one utilizing the traditional attenuated virus approach, could be less effective in older people than in younger ones.
Given the extreme urgency of developing vaccines that work well for everyone, we need to make sure that researchers are exploring all of the ways our elders can be best protected. While generating a vaccine that works equally well for people of all ages would be ideal, we can't count on that.
One way to bridge this gap might be to trick the bodies of older people into behaving as if they are younger just at the moment what a vaccine is delivered by giving them pre-immunization boosters.
Two recent phase 2 studies in older adults have suggested that a new category of drugs called rapalogues can in some cases increase the immunization capacity of older adults. Use of the drug for a short time period before flu shot immunization increased the antibody production for the flu and resulted in a 52 percent decrease in the occurrence of severe diseases needing medical help or hospitalization. This short-term pre-immunization intervention can also decrease the severity of serious respiratory tract infections, the deadliest manifestations of COVID-19, by similar magnitude. These patients also had almost half the incidence of the non-COVID-19 coronaviruses associated with the common cold.
The fact that those people were protected by treatment before hospitalization suggests metformin may have a role in boosting the vaccination of older people.
An inexpensive generic drug called metformin similarly targets the decline in immunity and inflammation (and extends health span and lifespan) in animals and has been used for decades to protect against the flu. A recent paper from a hospital in Wuhan, China showed that mortality of elderly COVID-19 diabetic patients on metformin was 25 percent less than that of patients with diabetes but not on metformin.
Another study from the U.S. showed that COVID-19 patients on metformin had a 20 percent decrease in mortality and lower inflammation. The fact that those people were protected by treatment before hospitalization suggests metformin may have a role in boosting the vaccination of older people.
We don't yet know whether rapalogues or metformin could be used as COVID-19 immunization boosters, not least because we don't have those vaccines. But we can and should make sure that all vaccine trials including older subjects also consider offering a subset of those subjects appropriate doses of rapalogues or metformin to explore whether doing so can boost the efficacy of a given vaccine.
If we weren't in the middle of the worst pandemic in a century, we would have more time to test our vaccines slowly and sequentially. In the context of the current crisis, however, testing whether immunization boosters might increase the efficacy of potential COVID-19 vaccines for older adults is at the very least a hypothesis worth exploring.