Beyond Henrietta Lacks: How the Law Has Denied Every American Ownership Rights to Their Own Cells

A 2017 portrait of Henrietta Lacks.
The common perception is that Henrietta Lacks was a victim of poverty and racism when in 1951 doctors took samples of her cervical cancer without her knowledge or permission and turned them into the world's first immortalized cell line, which they called HeLa. The cell line became a workhorse of biomedical research and facilitated the creation of medical treatments and cures worth untold billions of dollars. Neither Lacks nor her family ever received a penny of those riches.
But racism and poverty is not to blame for Lacks' exploitation—the reality is even worse. In fact all patients, then and now, regardless of social or economic status, have absolutely no right to cells that are taken from their bodies. Some have called this biological slavery.
How We Got Here
The case that established this legal precedent is Moore v. Regents of the University of California.
John Moore was diagnosed with hairy-cell leukemia in 1976 and his spleen was removed as part of standard treatment at the UCLA Medical Center. On initial examination his physician, David W. Golde, had discovered some unusual qualities to Moore's cells and made plans prior to the surgery to have the tissue saved for research rather than discarded as waste. That research began almost immediately.
"On both sides of the case, legal experts and cultural observers cautioned that ownership of a human body was the first step on the slippery slope to 'bioslavery.'"
Even after Moore moved to Seattle, Golde kept bringing him back to Los Angeles to collect additional samples of blood and tissue, saying it was part of his treatment. When Moore asked if the work could be done in Seattle, he was told no. Golde's charade even went so far as claiming to find a low-income subsidy to pay for Moore's flights and put him up in a ritzy hotel to get him to return to Los Angeles, while paying for those out of his own pocket.
Moore became suspicious when he was asked to sign new consent forms giving up all rights to his biological samples and he hired an attorney to look into the matter. It turned out that Golde had been lying to his patient all along; he had been collecting samples unnecessary to Moore's treatment and had turned them into a cell line that he and UCLA had patented and already collected millions of dollars in compensation. The market for the cell lines was estimated at $3 billion by 1990.
Moore felt he had been taken advantage of and filed suit to claim a share of the money that had been made off of his body. "On both sides of the case, legal experts and cultural observers cautioned that ownership of a human body was the first step on the slippery slope to 'bioslavery,'" wrote Priscilla Wald, a professor at Duke University whose career has focused on issues of medicine and culture. "Moore could be viewed as asking to commodify his own body part or be seen as the victim of the theft of his most private and inalienable information."
The case bounced around different levels of the court system with conflicting verdicts for nearly six years until the California Supreme Court ruled on July 9, 1990 that Moore had no legal rights to cells and tissue once they were removed from his body.
The court made a utilitarian argument that the cells had no value until scientists manipulated them in the lab. And it would be too burdensome for researchers to track individual donations and subsequent cell lines to assure that they had been ethically gathered and used. It would impinge on the free sharing of materials between scientists, slow research, and harm the public good that arose from such research.
"In effect, what Moore is asking us to do is impose a tort duty on scientists to investigate the consensual pedigree of each human cell sample used in research," the majority wrote. In other words, researchers don't need to ask any questions about the materials they are using.
One member of the court did not see it that way. In his dissent, Stanley Mosk raised the specter of slavery that "arises wherever scientists or industrialists claim, as defendants have here, the right to appropriate and exploit a patient's tissue for their sole economic benefit—the right, in other words, to freely mine or harvest valuable physical properties of the patient's body. … This is particularly true when, as here, the parties are not in equal bargaining positions."
Mosk also cited the appeals court decision that the majority overturned: "If this science has become for profit, then we fail to see any justification for excluding the patient from participation in those profits."
But the majority bought the arguments that Golde, UCLA, and the nascent biotechnology industry in California had made in amici briefs filed throughout the legal proceedings. The road was now cleared for them to develop products worth billions without having to worry about or share with the persons who provided the raw materials upon which their research was based.
Critical Views
Biomedical research requires a continuous and ever-growing supply of human materials for the foundation of its ongoing work. If an increasing number of patients come to feel as John Moore did, that the system is ripping them off, then they become much less likely to consent to use of their materials in future research.
Some legal and ethical scholars say that donors should be able to limit the types of research allowed for their tissues and researchers should be monitored to assure compliance with those agreements. For example, today it is commonplace for companies to certify that their clothing is not made by child labor, their coffee is grown under fair trade conditions, that food labeled kosher is properly handled. Should we ask any less of our pharmaceuticals than that the donors whose cells made such products possible have been treated honestly and fairly, and share in the financial bounty that comes from such drugs?
Protection of individual rights is a hallmark of the American legal system, says Lisa Ikemoto, a law professor at the University of California Davis. "Putting the needs of a generalized public over the interests of a few often rests on devaluation of the humanity of the few," she writes in a reimagined version of the Moore decision that upholds Moore's property claims to his excised cells. The commentary is in a chapter of a forthcoming book in the Feminist Judgment series, where authors may only use legal precedent in effect at the time of the original decision.
"Why is the law willing to confer property rights upon some while denying the same rights to others?" asks Radhika Rao, a professor at the University of California, Hastings College of the Law. "The researchers who invest intellectual capital and the companies and universities that invest financial capital are permitted to reap profits from human research, so why not those who provide the human capital in the form of their own bodies?" It might be seen as a kind of sweat equity where cash strapped patients make a valuable in kind contribution to the enterprise.
The Moore court also made a big deal about inhibiting the free exchange of samples between scientists. That has become much less the situation over the more than three decades since the decision was handed down. Ironically, this decision, as well as other laws and regulations, have since strengthened the power of patents in biomedicine and by doing so have increased secrecy and limited sharing.
"Although the research community theoretically endorses the sharing of research, in reality sharing is commonly compromised by the aggressive pursuit and defense of patents and by the use of licensing fees that hinder collaboration and development," Robert D. Truog, Harvard Medical School ethicist and colleagues wrote in 2012 in the journal Science. "We believe that measures are required to ensure that patients not bear all of the altruistic burden of promoting medical research."
Additionally, the increased complexity of research and the need for exacting standardization of materials has given rise to an industry that supplies certified chemical reagents, cell lines, and whole animals bred to have specific genetic traits to meet research needs. This has been more efficient for research and has helped to ensure that results from one lab can be reproduced in another.
The Court's rationale of fostering collaboration and free exchange of materials between researchers also has been undercut by the changing structure of that research. Big pharma has shrunk the size of its own research labs and over the last decade has worked out cooperative agreements with major research universities where the companies contribute to the research budget and in return have first dibs on any findings (and sometimes a share of patent rights) that come out of those university labs. It has had a chilling effect on the exchange of materials between universities.
Perhaps tracking cell line donors and use restrictions on those donations might have been burdensome to researchers when Moore was being litigated. Some labs probably still kept their cell line records on 3x5 index cards, computers were primarily expensive room-size behemoths with limited capacity, the internet barely existed, and there was no cloud storage.
But that was the dawn of a new technological age and standards have changed. Now cell lines are kept in state-of-the-art sub zero storage units, tagged with the source, type of tissue, date gathered and often other information. Adding a few more data fields and contacting the donor if and when appropriate does not seem likely to disrupt the research process, as the court asserted.
Forging the Future
"U.S. universities are awarded almost 3,000 patents each year. They earn more than $2 billion each year from patent royalties. Sharing a modest portion of these profits is a novel method for creating a greater sense of fairness in research relationships that we think is worth exploring," wrote Mark Yarborough, a bioethicist at the University of California Davis Medical School, and colleagues. That was penned nearly a decade ago and those numbers have only grown.
The Michigan BioTrust for Health might serve as a useful model in tackling some of these issues. Dried blood spots have been collected from all newborns for half a century to be tested for certain genetic diseases, but controversy arose when the huge archive of dried spots was used for other research projects. As a result, the state created a nonprofit organization to in essence become a biobank and manage access to these spots only for specific purposes, and also to share any revenue that might arise from that research.
"If there can be no property in a whole living person, does it stand to reason that there can be no property in any part of a living person? If there were, can it be said that this could equate to some sort of 'biological slavery'?" Irish ethicist Asim A. Sheikh wrote several years ago. "Any amount of effort spent pondering the issue of 'ownership' in human biological materials with existing law leaves more questions than answers."
Perhaps the biggest question will arise when -- not if but when -- it becomes possible to clone a human being. Would a human clone be a legal person or the property of those who created it? Current legal precedent points to it being the latter.
Today, October 4, is the 70th anniversary of Henrietta Lacks' death from cancer. Over those decades her immortalized cells have helped make possible miraculous advances in medicine and have had a role in generating billions of dollars in profits. Surviving family members have spoken many times about seeking a share of those profits in the name of social justice; they intend to file lawsuits today. Such cases will succeed or fail on their own merits. But regardless of their specific outcomes, one can hope that they spark a larger public discussion of the role of patients in the biomedical research enterprise and lead to establishing a legal and financial claim for their contributions toward the next generation of biomedical research.
Three Big Biotech Ideas to Watch in 2020—And Beyond
Body-on-a-chip, prime editing, and gut microbes all are poised to make a big impact in 2020.
1. Happening Now: Body-on-a-Chip Technology Is Enabling Safer Drug Trials and Better Cancer Research
Researchers have increasingly used the technology known as "lab-on-a-chip" or "organ-on-a-chip" to test the effects of pharmaceuticals, toxins, and chemicals on humans. Rather than testing on animals, which raises ethical concerns and can sometimes be inaccurate, and human-based clinical trials, which can be expensive and difficult to iterate, scientists turn to tiny, micro-engineered chips—about the size of a thumb drive.
It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip.
The chips are lined with living samples of human cells, which mimic the physiology and mechanical forces experienced by cells inside the human body, down to blood flow and breathing motions; the functions of organs ranging from kidneys and lungs to skin, eyes, and the blood-brain barrier.
A more recent—and potentially even more useful—development takes organ-on-a-chip technology to the next level by integrating several chips into a "body-on-a-chip." Since human organs don't work in isolation, seeing how they all react—and interact—once a foreign element has been introduced can be crucial to understanding how a certain treatment will or won't perform. Dr. Shyni Varghese, a MEDx investigator at the Duke University School of Medicine, is one of the researchers working with these systems in order to gain a more nuanced understanding of how multiple different organs react to the same stimuli.
Her lab is working on "tumor-on-a-chip" models, which can not only show the progression and treatment of cancer, but also model how other organs would react to immunotherapy and other drugs. "The effect of drugs on different organs can be tested to identify potential side effects," Varghese says. In addition, these models can help the researchers figure out how cancers grow and spread, as well as how to effectively encourage immune cells to move in and attack a tumor.
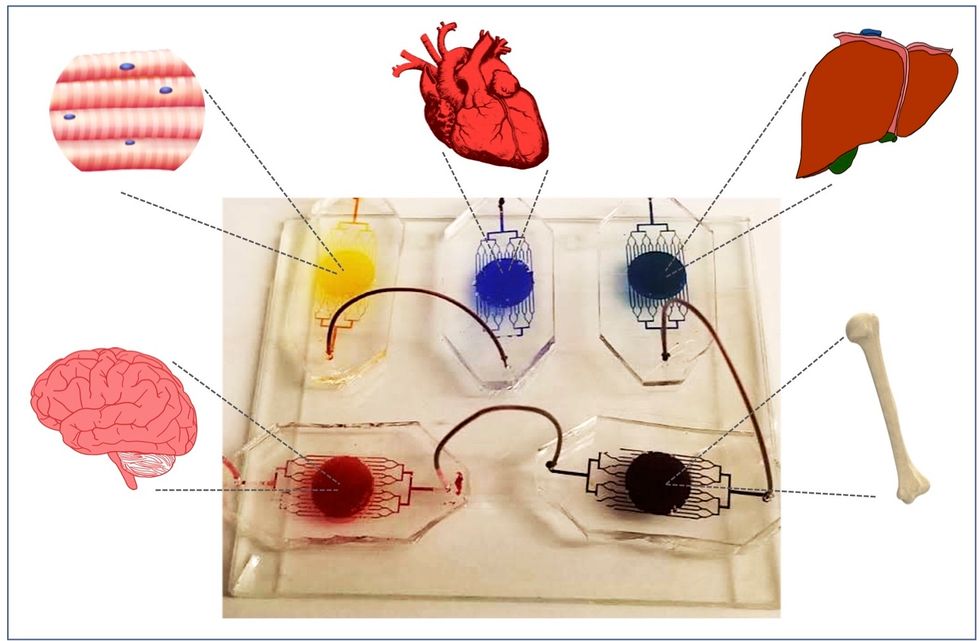
One body-on-a-chip used by Dr. Varghese's lab tracks the interactions of five organs—brain, heart, liver, muscle, and bone.
As their research progresses, Varghese and her team are looking for ways to maintain the long-term function of the engineered organs. In addition, she notes that this kind of research is not just useful for generalized testing; "organ-on-chip technologies allow patient-specific analyses, which can be used towards a fundamental understanding of disease progression," Varghese says. It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip for safety, efficacy, and potential side effects before writing a prescription.
2. Happening Soon: Prime Editing Will Have the Power to "Find and Replace" Disease-Causing Genes
Biochemist David Liu made industry-wide news last fall when he and his lab at MIT's Broad Institute, led by Andrew Anzalone, published a paper on prime editing: a new, more focused technology for editing genes. Prime editing is a descendant of the CRISPR-Cas9 system that researchers have been working with for years, and a cousin to Liu's previous innovation—base editing, which can make a limited number of changes to a single DNA letter at a time.
By contrast, prime editing has the potential to make much larger insertions and deletions; it also doesn't require the tweaked cells to divide in order to write the changes into the DNA, which could make it especially suitable for central nervous system diseases, like Parkinson's.
Crucially, the prime editing technique has a much higher efficiency rate than the older CRISPR system, and a much lower incidence of accidental insertions or deletions, which can make dangerous changes for a patient.
It also has a very broad potential range: according to Liu, 89% of the pathogenic mutations that have been collected in ClinVar (a public archive of human variations) could, in principle, be treated with prime editing—although he is careful to note that correcting a single genetic mutation may not be sufficient to fully treat a genetic disease.
Figuring out just how prime editing can be used most effectively and safely will be a long process, but it's already underway. The same day that Liu and his team posted their paper, they also made the basic prime editing constructs available for researchers around the world through Addgene, a plasmid repository, so that others in the scientific community can test out the technique for themselves. It might be years before human patients will see the results, and in the meantime, significant bioethical questions remain about the limits and sociological effects of such a powerful gene-editing tool. But in the long fight against genetic diseases, it's a huge step forward.
3. Happening When We Fund It: Focusing on Microbiome Health Could Help Us Tackle Social Inequality—And Vice Versa
The past decade has seen a growing awareness of the major role that the microbiome, the microbes present in our digestive tract, play in human health. Having a less-healthy microbiome is correlated with health risks like diabetes and depression, and interventions that target gut health, ranging from kombucha to fecal transplants, have cropped up with increasing frequency.
New research from the University of Maine's Dr. Suzanne Ishaq takes an even broader view, arguing that low-income and disadvantaged populations are less likely to have healthy, diverse gut bacteria, and that increasing access to beneficial microorganisms is an important juncture of social justice and public health.
"Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
"Typically, having a more diverse bacterial community is associated with health, and having fewer different species is associated with illness and may leave you open to infection from bacteria that are good at exploiting opportunities," Ishaq says.
Having a healthy biome doesn't mean meeting one fixed ratio of gut bacteria, since different combinations of microbes can generate roughly similar results when they work in concert. Generally, "good" microbes are the ones that break down fiber and create the byproducts that we use for energy, or ones like lactic acid bacteria that work to make microbials and keep other bacteria in check. The microbial universe in your gut is chaotic, Ishaq says. "Microbes in your gut interact with each other, with you, with your food, or maybe they don't interact at all and pass right through you." Overall, it's tricky to name specific microbial communities that will make or break someone's health.
There are important corollaries between environment and biome health, though, which Ishaq points out: Living in urban environments reduces microbial exposure, and losing the microorganisms that humans typically source from soil and plants can reduce our adaptive immunity and ability to fight off conditions like allergies and asthma. Access to green space within cities can counteract those effects, but in the U.S. that access varies along income, education, and racial lines. Likewise, lower-income communities are more likely to live in food deserts or areas where the cheapest, most convenient food options are monotonous and low in fiber, further reducing microbial diversity.
Ishaq also suggests other areas that would benefit from further study, like the correlation between paid family leave, breastfeeding, and gut microbiota. There are technical and ethical challenges to direct experimentation with human populations—but that's not what Ishaq sees as the main impediment to future research.
"The biggest roadblock is money, and the solution is also money," she says. "Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
That means investment in things we already understand to improve public health, like better education and healthcare, green space, and nutritious food. It also means funding ambitious, interdisciplinary research that will investigate the connections between urban infrastructure, housing policy, social equity, and the millions of microbes keeping us company day in and day out.
Scientists Just Started Testing a New Class of Drugs to Slow--and Even Reverse--Aging
Eliminating "zombie-like" cells, called senescent cells, may hold the key to slowing aging and its chronic diseases.
Imagine reversing the processes of aging. It's an age-old quest, and now a study from the Mayo Clinic may be the first ray of light in the dawn of that new era.
The immune system can handle a certain amount of senescence, but that capacity declines with age.
The small preliminary report, just nine patients, primarily looked at the safety and tolerability of the compounds used. But it also showed that a new class of small molecules called senolytics, which has proven to reverse markers of aging in animal studies, can work in humans.
Aging is a relentless assault of chronic diseases including Alzheimer's, cardiovascular disease, diabetes, and frailty. Developing one chronic condition strongly predicts the rapid onset of another. They pile on top of each other and impede the body's ability to respond to the next challenge.
"Potentially, by targeting fundamental aging processes, it may be possible to delay or prevent or alleviate multiple age-related conditions and many diseases as a group, instead of one at a time," says James Kirkland, the Mayo Clinic physician who led the study and is a top researcher in the growing field of geroscience, the biology of aging.
Getting Rid of "Zombie" Cells
One element common to many of the diseases is senescence, a kind of limbo or zombie-like state where cells no longer divide or perform many regular functions, but they don't die. Senescence is thought to be beneficial in that it inhibits the cancerous proliferation of cells. But in aging, the senescent cells still produce molecules that create inflammation both locally and throughout the body. It is a cycle that feeds upon itself, slowly ratcheting down normal body function and health.
Disease and harmful stimuli like radiation to treat cancer can also generate senescence, which is why young cancer patients seem to experience earlier and more rapid aging. The immune system can handle a certain amount of senescence, but that capacity declines with age. There also appears to be a threshold effect, a tipping point where senescence becomes a dominant factor in aging.
Kirkland's team used an artificial intelligence approach called machine learning to look for cell signaling networks that keep senescent cells from dying. To date, researchers have identified at least eight such signaling networks, some of which seem to be unique to a particular type of cell or tissue, but others are shared or overlap.
Then a computer search identified molecules known to disrupt these signaling pathways "and allow cells that are fully senescent to kill themselves," he explains. The process is a bit like looking for the right weapons in a video game to wipe out lingering zombie cells. But instead of swords, guns, and grenades, the list of biological tools so far includes experimental molecules, approved drugs, and natural supplements.
Treatment
"We found early on that targeting single components of those networks will only kill a very small minority of senescent cells or senescent cell types," says Kirkland. "So instead of going after one drug-one target-one disease, we're going after networks with combinations of drugs or drugs that have multiple targets. And we're going after every age-related disease."
The FDA is grappling with guidance for researchers wanting to conduct clinical trials on something as broad as aging rather than a single disease.
The large number of potential senolytic (i.e. zombie-neutralizing) compounds they identified allowed Kirkland to be choosy, "purposefully selecting drugs where the side effects profile was good...and with short elimination half-lives." The hit and run approach meant they didn't have to worry about maintaining a steady state of drugs in the body for an extended period of time. Some of the compounds they selected need only a half hour exposure to trigger the dying process in senescent cells, which can then take several days.
Work in mice has already shown impressive results in reversing diabetes, weight gain, Alzheimer's, cardiovascular disease and other conditions using senolytic agents.
That led to Kirkland's pilot study in humans with diabetes-related kidney disease using a three-day regimen of dasatinib, a kinase inhibitor first approved in 2006 to treat some forms of blood cancer, and quercetin, a flavonoid found in many plants and sold as a food supplement.
The combination was safe and well tolerated; it reduced the number of senescent cells in the belly fat of patients and restored their normal function, according to results published in September in the journal EBioMedicine. This preliminary paper was based on 9 patients in an ongoing study of 30 patients.
Kirkland cautions that these are initial and incomplete findings looking primarily at safety issues, not effectiveness. There is still much to be learned about the use of senolytics, starting with proof that they actually provide clinical benefit, and against what chronic conditions. The drug combinations, doses, duration, and frequency, not to mention potential risks all must be worked out. Additional studies of other diseases are being developed.
What's Next
Ron Kohanski, a senior administrator at the NIH National Institute on Aging (NIA), says the field of senolytics is so new that there isn't even a consensus on how to identify a senescent cell, and the FDA is grappling with guidance for researchers wanting to conduct clinical trials on something as broad as aging rather than a single disease.
Intellectual property concerns may temper the pharmaceutical industry's interest in developing senolytics to treat chronic diseases of aging. It looks like many mix-and-match combinations are possible, and many of the potential molecules identified so far are found in nature or are drugs whose patents have or will soon expire. So the ability to set high prices for such future drugs, and hence the willingness to spend money on expensive clinical trials, may be limited.
Still, Kohanski believes the field can move forward quickly because it often will include products that are already widely used and have a known safety profile. And approaches like Kirkland's hit and run strategy will minimize potential exposure and risk.
He says the NIA is going to support a number of clinical trials using these new approaches. Pharmaceutical companies may feel that they can develop a unique part of a senolytic combination regimen that will justify their investment. And if they don't, countries with socialized medicine may take the lead in supporting such research with the goal of reducing the costs of treating aging patients.

